Studies of living primates have shown how their diet determines many aspects of their lives, such as geographic range, body size, locomotor behaviour (how they moved) and breeding strategy. Diet must have occupied a similarly important place in the biology of extinct primates and therefore, if we are to understand the evolution of hominins (the grouping that includes our own species and extinct fossil ancestors), it important to try and uncover evidence for this past diet. The sources of information available for this vary between different parts of the fossil record. For later hominins, archaeological materials such as stone tools and butchered animal bones provide a key form of evidence with which to reconstruct their diet. Early hominin fossils (4.4 to 1.8 million years ago), however, are rarely associated with these types of evidence. Instead research into the early hominin diet has had to rely on alternative sources of evidence, including the size and shape of the skull and teeth (craniodental morphology); palaeoecology; dental microwear; and stable isotopes. While each of these different lines of evidence has given us important insights into past diets, they often provide conflicting results. In 2012, a new project began at the UCL Institute of Archaeology examining tooth wear patterns in low magnification images of early fossil hominins. This type of study is known as dental macrowear analysis and is often used to examine diet in archaeological populations, but this is the first time a detailed study has been applied to the early hominin fossil record.
The fossil record
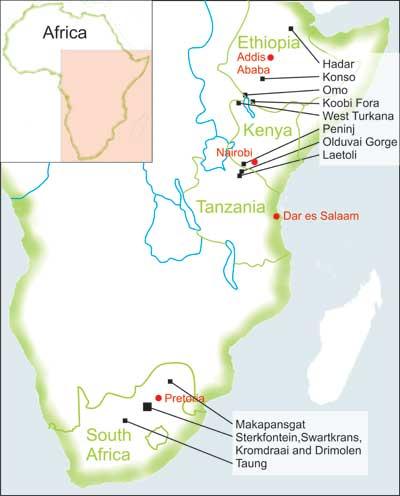
The early hominin fossil record is dominated by two main genera – Australopithecus and Paranthropus. These two groups are dated to a geological time period between the end of the Pliocene and beginning of the Pleistocene, known as the Plio-Pleistocene. Like most mammal fossils, they are especially well represented by jaws and teeth because the dental tissues are much more resistant than bone to most forms of physical and chemical attack during fossilization. Four different taxa (a group of related organisms) within these two main genera are particularly well known within the fossil record – Australopithecus afarensis and Paranthropus boisei from sites in East Africa and Paranthropus robustus and Australopithecus africanus from sites in South Africa (Fig. 1).
Craniodental evidence
Compared with most primates, both living and extinct, Australopithecus and Paranthropus had relatively large, flat, thick enamelled cheek teeth held within robust jaws with large muscle attachments (Robinson, 1956; Wolpoff, 1973; Rak, 1983; Wood and Abbot, 1983; Kay, 1985; Martin, 1985; Benyon and Wood, 1986; Macho and Thackery, 1992; Teaford and Ungar, 2000; Ungar, 2004; Lucas, 2007). These features have been interpreted as adaptations for masticating a mechanically resistant diet that demanded the generation of a powerful bite force over a broad grinding area of teeth (Kay, 1981; Walker, 1981; Demes and Creel, 1988; Hylander, 1988; Daegling and Grine, 1991; Teaford and Ungar, 2000; Strait et al., 2009). This diet is thought to have included hard, brittle foods such as nuts, fruits with hard shells, skins and stones, and possibly some soft foods such as flowers and buds (Kay, 1981; 1985; Lucas and Peters, 2000; Teaford and Ungar, 2000; Wood and Richmond 2000; Strait, et al., 2009). It has also been suggested that these early hominins would have had difficulty breaking down tough and more pliant food items like seed pods and meat (Lucas and Peters, 2000).
Australopithecus and Paranthropus have small anterior teeth (incisors and canines) relative to their cheek teeth (premolars and molars) in comparison with later hominins such as modern humans (Jolly, 1970; Kay, 1985; Ungar and Grine, 1991; Teaford and Ungar, 2000). This is interpreted as the result of less emphasis being placed on foods that required substantial incisor use, such as those with large thick husks and hard seeds surrounded by a thick layer of flesh (Teaford and Ungar, 2000).
Whilst these early hominins share common features in their craniodental morphologies, there are clear contrasts between them which suggest that their diets might have differed (Figs 2 and 3). Paranthropus specimens, in particular, have greatly enlarged molars and premolars relative to their tiny incisors and canines with exceedingly thick enamel crowns and massively proportioned jaws (Grine, 1981; Kay, 1981; 1985; Walker, 1981; Ungar, 2004). This suggests that they represented an even more specialised adaptation to hard foods, with increased grinding surfaces, greater abrasion resistance and stronger tooth supporting structures and musculature (Robinson, 1954; Grine, 1981; Rak, 1983; Kay, 1985; Hylander, 1988). As Paranthropus is found later in the Plio-Pleistocene fossil record than Australopithecus, which had smaller and less heavily built cheek teeth, this has been taken to suggest that hard, abrasive foods became increasingly important during this epoch of geological time. This is supported by palaeoenvironmental data which suggest that Australopithecus occupied relatively wooded habitats, while Paranthropus inhabited more open environments requiring increased dietary flexibility and the ability to process different types of food, including harder items (Vrba, 1975; Reed, 1997; Avery, 2001).
Dental microwear studies
Microscopic examination of wear pits and scratches on dental enamel (dental microwear) provides direct evidence of what a fossil taxon ate, by making comparisons with living primates. The first microwear studies of early hominins suggested that P. robustus consumed harder more brittle foods that required more variable chewing forces than A. africanus who consumed tougher, but softer fruits and/or leaves (Grine, 1981; 1986; 1987; Grine and Kay, 1988). More recent work on microwear patterns in A. afarensis and P. boisei challenges this simple dichotomy in diet between Australopithecus and Paranthropus, which suggests that, although A. afarensis may h ave been morphologically equipped to process hard brittle foods, it did not necessarily do so (Grine et al., 2006; Ungar et al., 2010). Similarly, P. boisei is characterised by the largest cheek teeth of any known hominin, backed by the most robust jaws, supporting structures and large areas of muscle attachment which imply an ability to consume mechanically challenging foods on a regular basis (Jolly, 1970; Kay, 1985; Teaford and Ungar, 2000). The results of recent microwear analysis, however, have shown no evidence that they consumed particularly hard or tough foods (Ungar et al., 2008). Scott et al.’s (2005) study found that P. robustus was also unlikely to have been a specialised hard object feeder and that it is more likely, that hard, brittle foods were only an occasional part of their diet.
The original microwear studies of early hominins were hindered by the difficulty of making repeatable observations with the methods of the time (Grine et al., 2002). Now more objective and repeatable approaches are available for studying these features, such as high resolution scanning of the worn surfaces and three-dimensional surface texture analysis (see Scott et al., 2005). However, some limitations still apply. Microwear is confined to little worn teeth of younger individuals who still possess substantial amounts of occlusal enamel. The number of specimens that preserve good ante-mortem microwear features in fossils of this age is greatly limited, reducing the sample size suitable for further analysis. Microwear features also only represent food eaten during the period immediately before death and this had become known as the ‘Last Supper Effect’ (Grine, 1986). In addition, microwear studies tend to compare a single tooth type between taxa – for example, either a first molar or incisor – rather than examining the relationship between different teeth in the dentitions of the fossils which are being compared.
Stable isotope studies
In contrast to many of the microwear studies, the results from stable carbon isotope analysis suggest greater similarities between early hominins in their diets. A. africanus, for example, appears to have eaten a highly variable diet mostly consisting of C3 foods (such as fruits and leaves from trees, bushes and shrubs or animals which consumed them), but also exploited C4 resources (such as tropical grasses and some sedges or again the animals feeding on them) (Sponheimer and Lee-Thorp, 1999; van der Merwe et al., 2003). This is surprising because the palaeoenvironmental evidence suggests C3 resources were more abundant than C4 in their habitat (Vrba, 1975; Reed, 1997; Avery, 2001). The results for P. robustus showed similar values to A. africanus (Lee-Thorp et al., 1994) despite evidence that C4 plants were more abundant in their habitats (Vrba, 1995; Reed, 1997). It also appears that the P. robustus values changed between seasons and years, suggesting that, rather than being dietary specialist, they ate a very variable diet (Sponheimer et al., 2006). P. boisei specimens give values that suggest consumption of more C4 rich foods, such as tropical grasses and sedges, than all other early hominins, which indicate that, rather than processing hard objects, their robust craniodental anatomy was an adaptation for processing large quantities of low-quality vegetation (van der Merwe et al., 2008; Cerling et al., 2011). While stable carbon isotope research has provided some interesting results it does have some limitations. One of the biggest problems is that it is currently unable to distinguish between diets as different as folivory, frugivory and carnivory because they are all based on C3 plants in forested ecosystems.
In order to understand as much as we can about early hominin diets all available sources of information need to be used. One area which has currently been under-researched is dental macrowear. Whilst observations of the dental macrowear in early fossil hominins have been made (Robinson, 1956; Wolpoff, 1973; Wallace, 1973; Grine, 1981), a systematic study has never been carried out.
Dental macrowear
Tooth wear is a complex process resulting from contact either between teeth or with foreign bodies in the mouth, such as food, materials, artefacts and grit (Molnar, 1972). Two main types of tooth wear can take place between teeth: occlusal and approximal. Occlusal wear occurs between the occlusal (grinding and cutting) surfaces of opposing teeth in the upper and lower dentition, whereas approximal wear occurs between neighbouring teeth in the same jaw, at their contact points. Most dental macrowear studies have focused on the difference in occlusal wear patterns in modern humans and between agriculturalists and hunter-gatherers (Molnar, 1971; Smith, 1972; Turner, 1979; Hinton, 1981; Smith, 1984; Kaifu, 1999; Eshed et al., 2006; Deter, 2009). Smith (1984), for example, found that while hunter-gatherers maintained relatively flat occlusal wear, agriculturalists exhibited more oblique wear patterns. These differences were attributed to a reduction in the toughness of food consumed by the agricultural groups. Work with recent hunter-gatherers has also suggested that diet played a strong role in the balance of tooth wear through the dentition, although this is overlain by their strikingly heavy use of teeth as tools (Clement et al., 2009; Clement and Hillson, 2012).
The diets of modern humans would, however, have greatly differed from those of early hominins. A better comparison therefore might be the living chimpanzees, gorillas and orang-utans, which show marked contrasts in diet matched by variation in tooth morphology (Kay, 1985; M’kirera and Ungar, 2003; Lucas, 2004), but little is known about their pattern of tooth wear. Chimpanzees also, to some extent, use their teeth as tools in preparing foods, for example in modifying sticks for food gathering (Boesch and Boesch, 1990), but this is nothing compared to the heavy and sustained use in human hunter-gatherers. It is a reasonable assumption that early hominins also used their teeth as tools to some extent, but this is impossible to substantiate archaeologically because there is no directly associated evidence of tools or even food remains.
Research Questions
This research project aims to address three main questions about the pattern of dental macrowear in early fossil hominins:
-
How variable is the wear pattern amongst members of the same taxonomic group of early hominins; does this change with increasing overall tooth wear; does it show differences between males and females in those fossil groups where sexual dimorphism is suggested?
-
Taking this variation into account, are there consistent differences between taxa? In particular, is there a relationship between such differences and the robustness and size of molars/premolars and the jaws?
-
How does the variation seen in early hominins compare with that of living chimpanzees, gorillas and orang-utans, as well as modern humans; are there consistent differences between species? Much more is known about the diet of extant primates, which will therefore provide an important comparison.
Methods
Our project uses a dental macrowear method. The method works on the progressive exposure of underlying dentine as the enamel of a tooth crown is worn away. Almost all living humans are exceptional amongst primates in having very slow rates of tooth wear. This is in contrast to recent hunter-gatherers, the people who made the Upper Palaeolithic cultures and early farmers, all of whom showed a rapid rate of tooth wear, as does every other living and extinct primate (e.g. Smith, 1984; Dean et al., 1992; Clement et al., 2009, 2012; Clement and Hillson, 2012). Under these conditions, dentine is exposed very rapidly after the teeth erupt into the mouth. We use image analysis software to measure both occlusal and approximal wear from digital images of the occlusal (biting) surfaces of teeth, prepared from photographs of original specimens where possible, or from casts or scans of good quality published photographs.
ImageJ image analysis software is used to measure the area of the occlusal surface and the area of exposed dentine (Fig. 4). A dentine proportion is then calculated by dividing the area of exposed dentine by the area of the occlusal surface. As wear progresses, this dentine proportion increases steadily. ImageJ is also used to measure the circumference of the occlusal surface and then the part of this circumference which is taken up by approximal attrition facets. An approximal proportion is then calculated by dividing this facet length by the circumference of the occlusal surface.
Overall tooth wear naturally increases with age and this makes it difficult to compare the state of wear in younger and older individuals whose age at death is not independently known. To remove age as a factor, we present the dentine proportion of each tooth as a ratio of the dentine proportion of another tooth from the same jaw (usually the permanent first molar is the reference tooth as this is the first to emerge and therefore the most worn, but this varies with preservation and with the question being asked). The resulting figure for each tooth is known as the wear ratio and this allows heavily worn dentitions to be compared directly with lightly worn because it represents the wear pattern rather than the amount of wear. This maximises the number of individuals that can be included in the study.
The fundamental principle of interpretation of these wear ratios is that the dental eruption sequence – the order in which different teeth in the dentition appear in the mouth – dictates the amount of time each tooth is exposed to wear. In modern humans the first molar is normally the first permanent tooth to erupt, around 6 years of age, whereas the third molar erupts significantly later in an individual’s life, during the late teens and early twenties. Thus, the first molar is exposed to considerably more wear (10–16 years) than the third molar. If we assume a constant rate of wear for all teeth then, at any age, it follows that earlier erupting teeth will exhibit the most advanced wear. If the rate of tooth wear is not constant for all teeth, then the degree of wear will not reflect the expected eruption sequence and this tells us that some other factor affected the evenness of the wear through the dentition. Our studies with dental casts of recent high wear rate hunter-gatherers for whom age was known at the time impressions were taken have shown that, although occlusal tooth wear increased with age, the balance between different tooth types in the dentition remained relatively constant (Clement and Hillson, 2012).
Our previous research has shown that this method can be applied to dentitions at all states of wear in original specimens, casts, photographs or scans of published images (Clement et al., 2009; 2012; Clement and Hillson, 2012) and therefore, unlike microwear and stable isotope studies, it is not limited by access to original specimens. This allows us to include the majority of tooth fossils so far recovered for the taxa of interest. In addition, this method is less time consuming than microwear methods and can therefore be applied to much larger assemblages of fossils. It can be applied equally to teeth of different morphology from different taxa. Our study of recent hunter-gatherer people has made it clear that the balance of this wear measure between molars, premolars, the canine and incisors shows subtle differences due to diet and use of teeth as tools (Clement et al., 2012; Clement and Hillson, 2012). We believe that a similar approach is appropriate to the very different question of diet and adaptation in extinct early hominins.
If differences in the relative size and morphology of molars, premolars, canines and incisors in early hominins are indeed adaptations to the requirements for processing diets of differing texture, then it is reasonable to suggest that the balance of wear between these tooth types would also be different. It is also important to consider the timing and pattern of dental development in different taxa, as the ages at which different teeth erupt into the mouth will have a considerable effect on the balance of wear between them. Living chimpanzees, gorillas and orang-utans show a number of key differences from ourselves in eruption timing and sequence of their teeth. The chimpanzee permanent dentition, for example, is complete by 11–12 years of age and their first molars erupt between 3 and 4 years (Nissen and Riesen, 1964). Their first incisors erupt around two years after the first molars, at about the age their second molars erupt, whereas in humans the first incisors erupt almost at the same age as the first molars. This needs to be taken into account when considering tooth wear patterns. Histological examination of enamel and dentine of fossil teeth has suggested that both Paranthropus and Australopithecus had short dental development sequences more similar to chimpanzees and gorillas than to ourselves, but differed from each other in the formation times of their incisors and canine (Bromage and Dean, 1985; Dean, 1985; 2010; Dean et al., 2001; Dean and Lucas, 2009). Remarkably rapid formation of incisors and canines has been reported for Paranthropus (Bromage and Dean, 1985; Benyon and Dean, 1988). All these differences in dental development timing, and thus eruption, should also be reflected in wear patterns.
Current and future research objectives
-
Catalogue digital images of A. africanus and P. robustus teeth. Digital images were taken of all original specimens of A. africanus and P. robustus that were available for study during data collection trips to the University of Witswatersrand in Johannesburg and the Ditsong Museum of Natural History, South Africa (Fig. 5). These included specimens from the sites of Kromdraii, Makapansgat, Sterkfontein, Swartkrans and Taung (Fig. 1). Published images of specimens unavailable for study were also compiled.
-
Catalogue digital images of A. afarensis and P. boisei teeth. A data collection trip was undertaken to the University of Arizona, USA, where a catalogue of digital images of original specimens and dental casts of A. afarensis and P. boisei were compiled. These images included specimens from the sites of Hadar and Omo, in Ethiopia, Laetoli, Olduvai Gorge and Peninj, in Tanzania, and Koobi Fora, in Kenya (Fig. 1). Scans of published images were also included in the catalogue for any further specimens of A. afarensis and P. boisei.
-
Catalogue digital images of chimpanzees, gorilla and orang-utans. Digital images were taken of a large sample of wild shot chimpanzees and gorillas from the collections at the Powell Cotton Museum, UK. A future data collection trip to the Natural History Museum, London is planned to photograph a comparative sample of orang-utans.
-
Analysis of early hominin tooth wear patterns. Tooth wear has been measured from all digital images of early hominins within the project’s catalogue and the aim is to measure all images of the extant primates by the end of July 2013. The next step in this research project will be to compare tooth wear patterns in A. afarensis and A. africanus with those of the more robust early hominins, P. boisei and P. robustus. The wear patterns of these four taxa of early hominins will then be compared to the wear patterns of gorillas, chimpanzees and orang-utans, taking into account the differences and similarities in eruption sequence and dental morphology. In addition, early hominin and extant primate wear patterns will be compared with the database of high wear rate modern humans already held at the UCL Institute of Archaeology, taking into account differences between age groups, sexes and diet.
-
Context of early hominin dental macrowear patterns. The final part of this project will be to analyse the patterns of tooth wear in the early fossil hominins within the context of the published literature on stable isotope studies, dental microwear analysis, craniodental morphology and palaeoecology.
Conclusion
The fossil record suggests that hominin dietary capabilities changed greatly during the Plio-Pleistocene. Developing a better understanding of these changes is vital in order to further our knowledge of the behaviour of our early hominin ancestors. The concept of using tooth wear, in association with the morphology of the teeth, to reconstruct the diet of extinct mammals has a long history in palaeontology. It continues to be an important focus of discussions on the diet of fossil hominins, but attention has focused on dental microwear; a systematic study of dental macrowear has never previously been made. This project will contribute in new ways to our understanding of the early hominin diet through the simplicity of its methods, as it can be applied to most of the fossils so far discovered for the taxa of interest as well as comparative material from chimpanzees, gorillas and orang-utans. The concentration on balance of tooth wear between different teeth in the dentition enables a direct comparison to be made between younger and older individuals with greatly differing overall extents of wear. This is a central difficulty for other investigations of tooth wear which limits the comparisons they can make. It also limits their ability to consider variation in wear within taxa. The significance of our contribution therefore comes from our ability to consider differences between taxa within the context of variation across the largest possible groups of fossils and recent primates.
Acknowledgements
The authors wish to thank Professor Bill Kimbel, Arizona State University, for supplying the images of A. afarensis from Hadar, Ethiopia, as well as access to casts of other early hominin fossils. We are also grateful to Dr Bernhard Zipfel from the University of Witwatersrand and Ms Stephany Potze from the Ditsong National Museum of Natural History for granting access to the South Africa fossils, and to Ms Angela Gill for allowing us to study the collections at the Powell Cotton Museum, UK. This research is funded by the Leverhulme Trust.
References
1 Avery, D M. (2001). The Plio-Pleistocene vegetation and climate of Sterkfontein and Swartkrans, South Africa, based on micro-mammals. Journal of Human Evolution 41 (2) : 113–132, DOI: http://dx.doi.org/10.1006/jhev.2001.0483
2 Beynon, A D; Dean, M C. (1988). Distinct dental development patterns in early fossil hominids. Nature 335 (6190) : 509–514, DOI: http://dx.doi.org/10.1038/335509a0
3 Benyon, A D; Wood, B A. (1986). Variation in enamel thickness and structure in East African Hominids. American Journal of Physical Anthropology 70 (2) : 177–193, DOI: http://dx.doi.org/10.1002/ajpa.1330700205
4 Boesch, C; Boesch, H. (1990). Tool use and tool making in wild chimpanzees. Folia Primatologica 54 (1–2) : 86–99, DOI: http://dx.doi.org/10.1159/000156428
5 Bromage, T G; Dean, M C. (1985). Re-evaluation of the age at death of immature fossil hominids. Nature 317 (6037) : 525–527, DOI: http://dx.doi.org/10.1038/317525a0
6 Cerling, T E; Mbua, E; Kirera, F M; Manthi, F K; Grine, F E; Leakey, M G; Sponheimer, M; Uno, K T. (2011). Diet of Paranthropus boisei in the early Pleistocene of East Africa. Proceedings of the National Academy of Sciences 108 (23) : 9337–9341, DOI: http://dx.doi.org/10.1073/pnas.1105808108
7 Clement, A; Hillson, S; de la Torre, I; Townsend, G. (2009). Tooth use in Aboriginal Australia. Archaeology International 11 : 37–40, DOI: http://dx.doi.org/10.5334/ai.1111
8 Clement, A F; Hillson, S W; Aiello, L C. (2012). Neanderthal tooth wear and the anterior dental loading hypothesis. Journal of Human Evolution 62 (3) : 367–76, DOI: http://dx.doi.org/10.1016/j.jhevol.2011.11.014
9 Clement, A F; Hillson, S W. (2012). Intrapopulation variation in macro tooth wear patterns – a case study from Igloolik, Canada. American Journal of Physical Anthropology 149 (4) : 517–24, DOI: http://dx.doi.org/10.1002/ajpa.22153
10 Daegling, D J; Grine, F E. (1991). Compact bone distribution and biomechanics of early hominid mandibles. American Journal of Physical Anthropology 86 (3) : 321–339, DOI: http://dx.doi.org/10.1002/ajpa.1330860302
11 Dean, M C. (1985). Variation in the developing root cone angle of the permanent mandibular teeth of modern man and certain fossil hominids. American Journal of Physical Anthropology 68 (2) : 233–238, DOI: http://dx.doi.org/10.1002/ajpa.1330680210
12 Dean, M C. (2010). Retrieving chronological age from dental remains of early fossil hominins to reconstruct human growth in the past. Philosophical Transactions of the Royal Society B: Biological Sciences 365 (1556) : 3397–3410, DOI: http://dx.doi.org/10.1098/rstb.2010.0052
13 Dean, M C; Lucas, V S. (2009). Dental and skeletal growth in early fossil hominins. Annals of Human Biology 36 (5) : 545–561, DOI: http://dx.doi.org/10.1080/03014460902956725
14 Dean, M C; Jones, M E; Pilley, J R. (1992). The natural history of tooth wear, continuous eruption and periodontal disease in wild shot great apes. Journal of Human Evolution 22 (1) : 23–39, DOI: http://dx.doi.org/10.1016/0047-2484(92)90027-7
15 Dean, M C; Leakey, M G; Reid, D J; Schrenk, F; Schwartz, G T; Stringer, C; Walker, A. (2001). Growth processes in teeth distinguish modern humans from Homo erectus and earlier hominins. Nature 414 (6864) : 628–631, DOI: http://dx.doi.org/10.1038/414628a
16 Demes, B; Creel, N. (1988). Bite force, diet and cranial morphology of fossil hominids. Journal of Human Evolution 17 (7) : 657–670, DOI: http://dx.doi.org/10.1016/0047-2484(88)90023-1
17 Deter, C. (2009). Gradients of occlusal wear in hunter-gatherers and agriculturalists. American Journal of Physical Anthropology 138 : 247–254, DOI: http://dx.doi.org/10.1002/ajpa.20922
18 Eshed, V; Gopher, A; Hershkovitz, I. (2006). Tooth wear and dental pathology at the advent of agriculture: new evidence from the Levant. American Journal of Physical Anthropology 130 : 145–159, DOI: http://dx.doi.org/10.1002/ajpa.20362
19 Grine, F E. (1981). Trophic differences between ‘gracile’ and ‘robust’ australopithecines: a scanning electron microscope analysis of occlusal events. South African Journal of Science 77 (5) : 203–230.
20 Grine, F E. (1986). Dental evidence for dietary differences in Australopithecus and Paranthropus: a quantitative analysis of permanent molar microwear. Journal of Human Evolution 15 (8) : 783–822, DOI: http://dx.doi.org/10.1016/S0047-2484(86)80010-0
21 Grine, F E. (1987). Quantitative analysis of occlusal microwear in Australopithecus and Paranthropus. Scanning Microscopy 1 (2) : 647–656.
22 Grine, F E; Kay, R F. (1988). Early hominid diets from quantitative image analysis of dental microwear. Nature 333 (6175) : 765–768, DOI: http://dx.doi.org/10.1038/333765a0
23 Grine, F E; Ungar, P S; Teaford, M F. (2002). Error rates in dental microwear quantification using SEM. Scanning 24 (3) : 144–153, DOI: http://dx.doi.org/10.1002/sca.4950240307
24 Grine, F E; Ungar, P S; Teaford, M F; El-Zaatari, S. (2006). Molar microwear in Praeanthropus afarensis:evidence for dietary stasis through time and under diverse paleoecological conditions. Journal of Human Evolution 51 (3) : 297–319, DOI: http://dx.doi.org/10.1016/j.jhevol.2006.04.004
25 Hinton, R J. (1981). Form and patterning of anterior tooth wear among aboriginal human groups. American Journal Physical Anthropology 54 (4) : 555–564, DOI: http://dx.doi.org/10.1002/ajpa.1330540409
26 Hylander, W L. (1988). Implications of in vivo experiments for interpreting the functional significance of ‘robust’ australopithecine jaws In: Grine, F E (ed.), Evolutionary History of the ‘Robust’ Australopithecines. New York: CQU Press, pp. 55–83.
27 Jolly, C J. (1970). The seed-eaters: a new model of hominid evolution based on a baboon analogy. Man 5 (1) : 1–26.
28 Kaifu, Y. (1999). Changes in the pattern of tooth wear from prehistoric to recent periods in Japan. American Journal Physical Anthropology 109 (4) : 485–499, DOI: http://dx.doi.org/10.1002/(SICI)1096-8644(199908)109:4<485::AID-AJPA5>3.0.CO;2-K
29 Kay, R F. (1981). The nut-crackers – a new theory of the adaptations of the Ramapithecinae. American Journal of Physical Anthropology 55 (2) : 141–151, DOI: http://dx.doi.org/10.1002/ajpa.1330550202
30 Kay, R F. (1985). Dental evidence for the diet of Australopithecus. Annual Review of Anthropology 14 : 315–341, DOI: http://dx.doi.org/10.1146/annurev.an.14.100185.001531
31 Lee-Thorp, J A; van de Merwe, N J; Brain, C K. (1994). Diet of Australopithecus robustus at Swartkrans from stable carbon isotope analysis. Journal of Human Evolution 27 (4) : 361–372, DOI: http://dx.doi.org/10.1006/jhev.1994.1050
32 Lucas, P W; Peters, C R. (2000). Function of postcanine tooth crown shape in mammals In: Teaford, M F, Smith, M M; M M and Ferguson, M W J M W J (eds.), Development, Function and Evolution of Teeth. Cambridge: Cambridge University Press, pp. 283–289.
33 Lucas, P W. (2004). Dental Functional Morphology: How Teeth Work. New York: Cambridge University Press.
34 Lucas, P W. (2007). The evolution of the hominin diet from a dental functional perspective In: Ungar, P A (ed.), Evolution of the Human Diet: the Known, the Unknown and the Unknowable. Oxford: Oxford University Press, pp. 31–38.
35 Macho, G A; Thackeray, J F. (1992). Computer tomography and enamel thickness of maxillary molars of Plio-Pliocene hominids from Sterkfontein, Swartkrans and Kromdraai (South Africa): An exploratory study. American Journal of Physical Anthropology 89 (2) : 133–143, DOI: http://dx.doi.org/10.1002/ajpa.1330890202
36 Martin, L. (1985). Significance of enamel thickness in hominoid evolution. Nature 314 (6008) : 260–263, DOI: http://dx.doi.org/10.1038/314260a0
37 M’kirera, F; Ungar, P S. (2003). Occlusal relief changes with molar wear in Pan troglodytes and Gorilla gorilla gorilla. American Journal of Primatology 60 (2) : 41–41, DOI: http://dx.doi.org/10.1002/ajp.10077
38 Molnar, S. (1971). Human tooth wear, tooth function and cultural variability. American Journal of Physical Anthropology 34 (2) : 175–89, DOI: http://dx.doi.org/10.1002/ajpa.1330340204
39 Molnar, S. (1972). Tooth wear and culture: a survey of tooth functions among some prehistoric populations. Current Anthropology 13 (5) : 511–526, DOI: http://dx.doi.org/10.2307/2741004
40 van de Merwe, N J; Masao, F T; Bamford, M K. (2008). Isotopic evidence for contrasting diets of early hominins Homo habilis and Australopithecus boisei of Tanzania. South African Journal of Science 104 : 153–155.
41 van der Merwe, N J; Thackeray, J F; Lee-Thorp, J A; Luyt, J. (2003). The carbon isotope ecology and diet of Australopithecus africanus at Sterkfontein, South Africa. Journal of Human Evolution 44 (5) : 581–597, DOI: http://dx.doi.org/10.1016/S0047-2484(03)00050-2
42 Nissen, H W; Riesen, A H. (1964). The eruption of the permanent dentition of chimpanzee. American Journal of Physical Anthropology 22 (3) : 285–294, DOI: http://dx.doi.org/10.1002/ajpa.1330220315
43 Rak, Y. (1983). The Australopithecine Face. London: Academic Press.
44 Reed, K E. (1997). Early hominid evolution and ecological change through the African Plio-Pleistocene. Journal of Human Evolution 32 (2–3) : 289–322, DOI: http://dx.doi.org/10.1006/jhev.1996.0106
45 Robinson, J G. (1954). Prehominid dentition and hominid evolution. Evolution 8 : 324–334.
46 Robinson, J G. (1956). The dentition of the australopithecines. Memoirs of the Transvaal Museum 9 : 1–179.
47 Scott, R S; Ungar, P S; Bergstrom, T S; Brown, C A; Grine, F E; Teaford, M F; Walker, A. (2005). Dental microwear texture analysis shows within diet variability in fossil hominins. Nature 436 (7051) : 693–695, DOI: http://dx.doi.org/10.1038/nature03822
48 Smith, P. (1972). Diet and attrition in the Natufians. American Journal of Physical Anthropology 37 (2) : 233–238, DOI: http://dx.doi.org/10.1002/ajpa.1330370207
49 Smith, B H. (1984). Patterns of molar wear in hunter-gatherers and agriculturalists. American Journal of Physical Anthropology 63 (1) : 39–56, DOI: http://dx.doi.org/10.1002/ajpa.1330630107
50 Sponheimer, M; Lee-Thorp, J A. (1999). Isotopic Evidence for the Diet of an Early Hominin, Australopithecus africanus. Science 283 (5400) : 368–370, DOI: http://dx.doi.org/10.1126/science.283.5400.368
51 Sponheimer, M; Passey, B H; de Ruiter, D J; Guatelli-Steinberg, D; Cerling, T E; Lee-Thorp, J A. (2006). Isotopic evidence for dietary variability in the early hominin Paranthropus robustus. Science 314 (5801) : 980–982, DOI: http://dx.doi.org/10.1126/science.1133827
52 Strait, D S; Weber, G W; Neubauerm, S. (2009). The feeding biomechanics and dietary ecology of Australopithecus africanus. Proceedings of the National Academy of Sciences, USA 106 (7) : 2124–2129, DOI: http://dx.doi.org/10.1073/pnas.0808730106
53 Teaford, M F; Ungar, P S. (2000). Diet and the evolution of the earliest human ancestors. Proceedings of the National Academy of Sciences, USA 97 (25) : 13506–13511, DOI: http://dx.doi.org/10.1073/pnas.260368897
54 Turner, CG. (1979). Dental anthropological indications of agriculture among the Jomon people of central Japan. American Journal of Physical Anthropology 51 (4) : 619–636, DOI: http://dx.doi.org/10.1002/ajpa.1330510413
55 Ungar, P S. (2004). Dental topography and diets of Australopithecus afarensis and early Homo. Journal of Human Evolution 46 (5) : 605–622, DOI: http://dx.doi.org/10.1016/j.jhevol.2004.03.004
56 Ungar, P S; Grine, F E. (1991). Incisor size and wear in Australopithecus africanus and Paranthropus robustus. Journal of Human Evolution 20 (4) : 313–340, DOI: http://dx.doi.org/10.1016/0047-2484(91)90013-L
57 Ungar, P S; Grine, F E; Teaford, M F. (2008). Dental microwear and diet of Plio-Pleistocene hominin Paranthropus boisei. PLoS One 3 (4) : e2044. DOI: http://dx.doi.org/10.1371/journal.pone.0002044
58 Ungar, P S; Scott, R S; Grine, F E; Teaford, M F. (2010). Molar microwear textures and the diets of Australopithecus anamensis and Australopithecus afarensis. Philosophical Transactions of the Royal Society B: Biological Sciences 365 (1556) : 3345–3354, DOI: http://dx.doi.org/10.1098/rstb.2010.0033
59 Vrba, E S. (1975). Some evidence of chronology and palaeoecology of Sterkfontein, Swartkrans and Kromdraai from the fossil Bovidae. Nature 254 (5498) : 301–304, DOI: http://dx.doi.org/10.1038/254301a0
60 Vrba, E S. (1995). On the connections between paleoclimate and evolution In: Vrba, E S, Denton, G H; G H and Partridge, T C; T C, Burckle, L H L H (eds.), Paleoclimate and Evolution, with Emphasis on Human Origins. New Haven: Yale University Press, pp. 24–45.
61 Walker, A. (1981). Dietary Hypotheses and Human Evolution. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences 292 (1057) : 57–64, DOI: http://dx.doi.org/10.1098/rstb.1981.0013
62 Wallace, J A. (1973). Tooth chipping in the australopithecines. Nature 244 (5411) : 117–118, DOI: http://dx.doi.org/10.1038/244117a0
63 Wolpoff, M H. (1973). Posterior tooth size, body size, and diet in South African gracile Australopithecus. American Journal of Physical Anthropology 39 (3) : 375–394, DOI: http://dx.doi.org/10.1002/ajpa.1330390306
64 Wood, B A; Abbott, S A. (1983). Analysis of the dental morphology of Plio-Pleistocene hominids. I. Mandibular molars: crown area measurements and morphological traits. Journal of Anatomy 136 (1) : 197–219.
65 Wood, B; Richmond, B G. (2000). Human evolution: taxonomy and paleobiology. Journal of Anatomy 197 (1) : 19–60, DOI: http://dx.doi.org/10.1046/j.1469-7580.2000.19710019.x