Introduction
Indoor fungal growth may affect the health of occupants [1,2], disturb their comfort and well-being and lead to damage to the building fabric [3,4]. Therefore, it is of critical importance to be able to measure the extent of fungal growth correctly in a given indoor environment. Through testing, researchers aim to quantify fungal biomass, determine the conditions under which fungi flourish and assess whether the property needs remediation [5]. However, although many sampling and analysis techniques have been proposed and widely implemented [6], indoor fungal testing has not yet been fully standardised through well-established protocols [7,8].
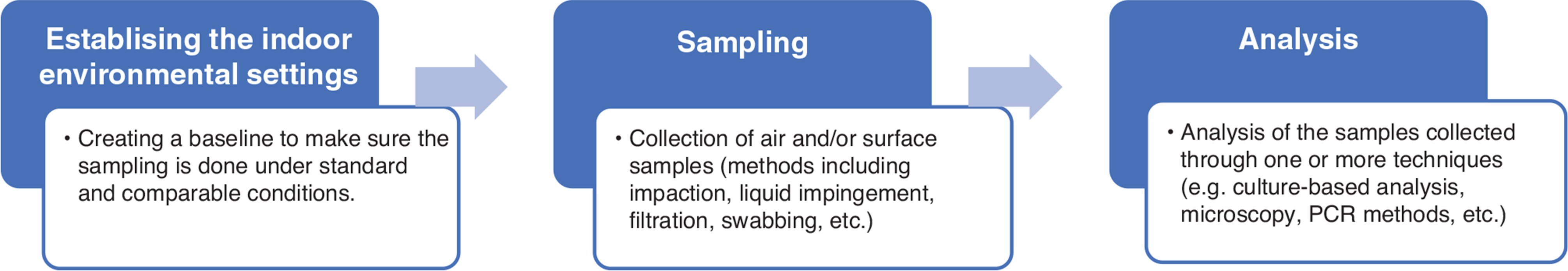
Indoor fungal testing protocols are typically composed of the following steps: (1) establishing environmental settings prior to sampling, (2) collection of samples and (3) sample analysis via one or more techniques to estimate the amount and/or the contents of indoor fungal biota (Fig. 1).
The first stage of testing includes all the activities carried out prior to sampling, that is, the establishment of the indoor environmental settings. The preparation may be done by creating a completely still environment by restricting any access to, or movements in, the spaces to be tested for some time prior to testing (termed as non-activated sampling), or by resuspending particles through mechanical means such as an air blower for a predetermined amount of time, often defined as a function of the room surface area or volume (termed as activated sampling). Between these two extremes are the protocols where the equipment setup and some movement of the occupants or the investigators before the testing are allowed (Table 1).
Some examples of non-activated (a, b) and activated protocols (c) (length of line segments not indicative of the durations associated with their labelled activities relative to each other)
| Examples of different protocols | Example studies |
|---|---|
|
Terčelj et al. [9]: Windows and doors were closed for several hours prior to sampling Shinohara et al. [10]: Tests were carried out in unoccupied spaces |

|
Gent et al. [11]; Dallongeville et al. [12]; Cahna et al. [13]; Nieto-Caballero et al. [14]: Activities before sampling were not restricted. The rooms were occupied before and during the sampling but no mechanical activation was carried out |

|
Aktas et al. [7,15,16]: Mechanical activation for approx. 1 min/10 m2 was carried out after the equipment setup. Upon completion of the activation, sampling was initiated |
-
(Source: Authors, 2022.)
As seen, while there is substantial literature on the selection of sampling and analysis techniques depending on the aim of the investigation and the availability of tools [5], the standardisation of protocols with regard to the preparation of the indoor environment prior to sampling has gained limited attention and interest [17,18,19]. This situation manifests as additional complexity when comparing and contrasting the findings of multiple research studies, even when the same sampling and analysis techniques are used.
Non-activated (non-aggressive) and activated (aggressive) protocols
The preparation of the environmental settings prior to sampling is often described by different and conflicting terms used interchangeably and without sufficiently clear definitions in the relevant literature, which leads to confusion. That is why a terminological discussion to supplement the work presented here was deemed necessary: the most common terms are active, activated and aggressive versus passive, non-activated and non-aggressive sampling to discriminate between resuspending the air, or not, before sampling [20]. Aktas et al. [7,15,16], Heinsohn [5] and Christensen and Swaebly [21] used the term active sampling to describe the collection of airborne particles after the disturbance of the air through artificial means, while passive sampling is used to describe the collection of particles from still air. Other researchers have used the same terms to differentiate between air sampling methods that require the creation of an artificial airflow for the collection of particles (e.g., impaction, liquid impingement, filtration and electrostatic precipitation) and methods that utilise gravitational settling (e.g., sedimentation), respectively [22–24]. To avoid common confusion over this overlap, in this paper we use the terms activated (aggressive) and non-activated (non-aggressive) to indicate still and perturbed indoor air conditions prior to sampling.
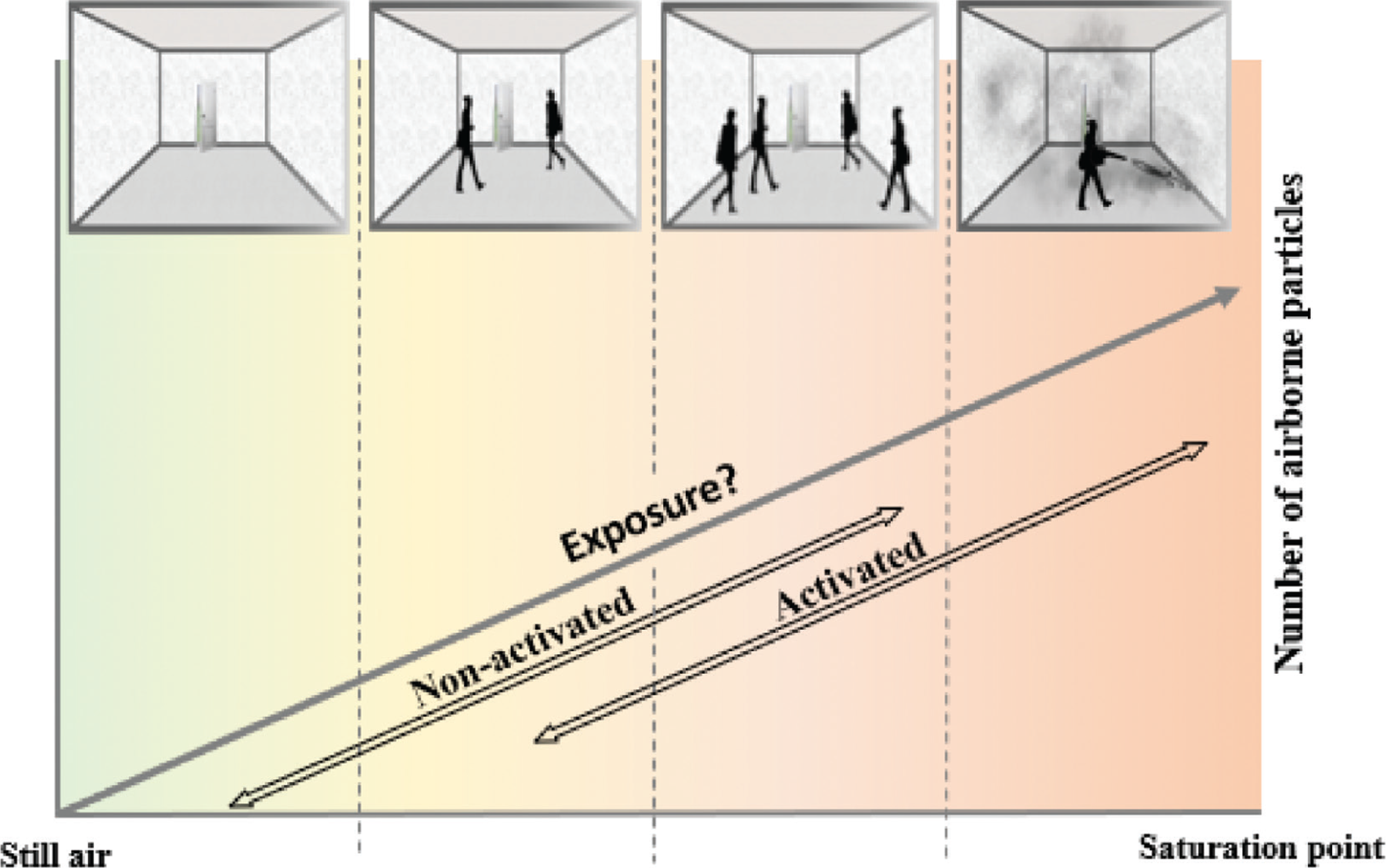
Terminological discourse aside, the boundary between what is considered to be still or actively mixed air is also often unclear, which has particularly dire implications for studies focussed on exposure (Fig. 2). Cahna et al. [11] and Nieto-Caballero et al. [14] collected air samples for the assessment of fungal contamination in classrooms while occupied by children. In another study by Shinohara et al. [10], house dust samples were collected from unoccupied houses in Japan, however there was no waiting time between equipment set-up and sampling. Aktas et al. [15] used a hand-held blower for 1 min/10 m2 to resuspend particles prior to sampling. In all three cases, the environmental settings under which the air sampling was carried out were different and the findings were not comparable. Although the use of a blower in the last example might be best placed to reach the ‘saturation point’ and can therefore be safely termed as an activated testing exercise (Fig. 2), where the other two cases sit within the wide spectrum of different levels of air stillness is unclear.
Effect of activities and environmental context on the sampling readings
Many researchers have reported that the level and nature of the activities within a given indoor space do influence the extent and rate of resuspension of bioaerosols, including the fungal matter (Table 2). The creation of artificial air currents due to the disturbance of the air’s steadiness was found to increase the concentration of the airborne fungi through resuspension, hence allowing the detection of particles that are otherwise not detectable [15,26]. However, how the readings obtained from non-activated and activated protocols differ is yet to be fully quantified. Importantly, the relevant literature is dominated by the use of non-activated protocols [12,13,32,33]. This is primarily on the claim that the collection of samples from still air can reduce reproducibility-related errors [5], although several studies using both protocols comparatively suggest otherwise [7,15,28].
Effect of the activation to the particle resuspension and fungal readings reported in the literature
| Research by | Context/sampling and analysis method | Outcomes |
|---|---|---|
| Flannigan [25] | Fieldwork/impaction and colony enumeration | Suggested that during the occupied hours, the human activity led to a significant increase in the resuspension of fungal particles in the restaurant tested leading to an increase in the viable fungal counts (approx. 16 times higher than the readings during unoccupied hours) |
| Rylander [26] | Fieldwork/filtration and NAHA measurements | Reported that the indoor fungal levels (NAHA levels) in rooms in villas and apartments with no mechanical ventilation increased up to 10 times when an activated protocol using a blower was implemented compared to the readings when a non-activated protocol was selected |
| Aktas et al. [15] | Fieldwork/impaction on agar plates and colony enumeration and filtration and NAHA detection | Reported an up to 56 times increase of the activated fungal readings compared to non-activated readings when testing was carried out in residential buildings in North London |
| Mundt [27] | Laboratory experiments/particle counters used to measure airborne particle concentrations | Reported an up to 10 times increase in the concentration of PM5 and PM10 when a person entered, and vigorously walked around the test room |
| Mukai et al. [28] | Laboratory experiments/particle counters used to measure airborne particle concentrations | Reported an up to 10 times increase of the relative resuspension rate of particles ranging from 0.5 to 20 μm when the turbulence intensity and air velocity directly above three substrates (linoleum, metal and carpet) increased by 23% and 20 m/s, respectively |
| Goldasteh et al. [29] | Laboratory experiments/particle counters used to measure airborne particle concentrations | Reported 1) approximately 10 times higher resuspension rates of 1–10 μm particle from hardwood flooring and 2) approximately 2–3 times higher resuspension rate of 1–10 μm from linoleum flooring when airflow speed increased from 4.5 to 21 m/s in a laminar flow wind tunnel |
| Napoli et al. [30] | Fieldwork/impaction and total viable counts (TVC enumeration) | Reported an increase of the mean TVC by approximately 7.5 times during operations in hospital rooms in the Apulia Region in South-eastern Italy |
| Wang et al. [31] | Fieldwork/particle counters used to measure airborne particle concentrations | Reported approximately 5.5 times increase in average PM2.5 concentration when the occupancy density increased from 10 to 35 people in a classroom of Jinnan Campus of Nankai University |
-
(Source: Authors, 2022.)
There are a number of parameters which would define the nature and extent of activation and would impact the readings in conjunction with it. The first and most immediate one is the changes in the pattern and the speed of the inspector’s movement across space which may lead to the resuspension of particles from different locations, potentially contaminated by fungi. Findings by Rylander [26] indicate that the movement of inspectors inside residential buildings prior to testing increased the measured concentration of fungal biota by two times compared to when all movement was strictly prohibited. Small-scale experiments and computational fluid dynamics (CFD) tests carried out by Cao et al. [34] indicated that human movement within a room could affect the dispersion of particles and that increase in the movement’s velocity is likely to lead to longer resuspension of fine particles (0.02–1 μm).
Another important parameter is the effect of the room characteristics/condition. Room characteristics, especially the level of cleanliness, can be connected to the amount of dust within a property and abiotic factors such as water and nutrient availability affecting the sporulation of certain fungal species [7,15,16,17]. In different properties, the quantity of dust and the fungi stored in it can vary. The volatility of fungi in dust can also change [35]. Hence, the particle aerosolisation rates may vary due to the different dust levels and volatility of fungal particles in every space and thus potential underestimation/overestimation issues regarding fungal readings may rise. Another important concern regarding the detectability of fungal particles, especially the larger ones, is how quickly the airborne fungal levels return to the pre-activation levels. Activities may lead to different levels of resuspension of particles of different sizes. Allowing a long time to pass after the end of the resuspension may lead to the settlement of heavy particles before they can be collected through sampling [36]. Mundt [27] suggests that large particles are expected to settle faster than small ones once indoor activities have stopped. To that end, the inability to specify the duration of time needed between the preparation of the indoor environment and the sampling might lead to variations in the sampling results.
Finally, another concern with regard to both the comparability of the testing outcomes from the available testing procedures and their ability to assess the appropriate potential fungal reservoirs is the uncertain impact of the hygrothermal conditions on the readings. The relative humidity affects the fungal growth and sporulation [37–39] directly but can also indirectly influence the resuspension rates and the recovery efficiency of fungal particles. Depending on the fungal species present the optimal relative humidity is likely to increase the spore production rate and conidial growth [37–39]. However, the moisture content in the indoor environment and the indoor materials can also affect the adhesion of fungi on the indoor aces and may vary across different spaces [40]. In high humidity conditions, liquid molecules can be adsorbed on small-sized bioaerosols, including small-sized (>0.1 μm) fungal particles leading to an increase in the adhesion forces between the particles and the surfaces they come in contact with [41]. As a result, the aerosolisation rate of particles by implementing the testing methods may vary from case to case, thus influencing the sampling readings.
We, therefore, raise the following open research question: What environmental settings prior to sampling ensure that the testing procedure is replicable, and lead to comparable results from different properties? This paper aims to fill this gap by detailing the impact of the indoor environmental settings prior to the sampling on the testing outcomes through a critical review of the literature (Materials and methods), a series of laboratory experiments and a case study demonstration (Results) to then discuss research and knowledge gaps, and implications on the practice (Discussion) and draw conclusions (Conclusions).
Materials and methods
Experimental work and a case study testing demonstration were performed to examine the effect of the environmental settings on the resuspension rates of particles including fungal ones. Different protocols utilising air activation via the resuspension of particles with a leaf blower were implemented in both segments of the study. While the experimental work aimed to test the effect of different activated protocols on the concentration of airborne particles of different sizes in a controlled environment, the case study was designed to examine the applicability and validity of the experimental outcomes in real-life scenarios.
Laboratory experiments
In order to investigate, on a quantitative basis, the impact of the level of ‘activation’ on the obtained readings, an experimental campaign was designed, and three sets of experiments were carried out. The experimental work aimed to identify the role of the blowing duration in the particle counts at different heights. The findings of this experimental work are then discussed in conjunction with the literature. For these experiments, particle counter readings were chosen as a proxy for indoor mould testing outcomes. This was considered a robust approach as fungal particles can range in size from 0.6 μm to 10 μm [42] and associations between the airborne fungal concentration changes and particle counts have been previously reported in the literature [43,44]. No cultivation and aerosolisation of fungal particles were carried out to minimise the risk of accidentally contaminating the test space.
The work was conducted in an environmental chamber (2.8 × 3 × 3 m) where particle counters were positioned at three different heights (Fig. 3) – the readings from six particle size channels were analysed before and after activation. To ensure that dust or small particles cannot infiltrate the room through cracks and openings, the environmental chamber joints were entirely air-sealed with sealing tape typically used to perform blow-door tests. It is important to mention that the ventilation system in the chamber was not operated at any instance during these tests, and the ducts leading to the chamber were also sealed to avoid unintended disturbance in the air.
Three particle counters (PMS 5003 by Plantower [Nanchang City, Jiangxi Province, China], sensitivity: 50% – 0.3 μm, 98% – 0.5 μm and larger; resolution: 1 μg/m3; work temperature: operating temperature −10°C to 60°C, humidity (work): 0–99%) were placed at heights of 0.75 m, 1.5 m and 2.25 m on a vertical pole located in the centre of the room to monitor the variation of different sized particles with height. All three sensors were calibrated by the manufacturer prior to use.
To implement activated protocols within the environmental chamber, a leaf blower (Model 100760 Merry Tools’ [Katsu Tools, Harrow, UK] air leaf dust blower electric inflator) was used. The blower was placed on top of a Bluetooth-operated slider (Neewer’s [Shenzhen, China] motorised camera slider, maximum travel distance: 1 m) and was connected to a Wi-Fi-enabled plug to ensure that it could be operated remotely, without entering the chamber. The blower was set at a fixed height (distance between nozzle end and wall = 1.3 m) and a fixed distance from the wall (distance between nozzle end and wall = 1.5 m) and was able to move horizontally for a distance of 1 m (Fig. 4).
Experiments were conducted to examine the effect of six different blowing durations on the concentration of particles at the three different heights in the centre of the room. Tests were repeated three times (Series 1, 2, and 3 in Fig. 6) to reduce the likelihood of anomalous results and increase the accuracy. The blowing durations were set to be 1 min, 2 min, 3 min, 5 min, 10 min and 15 min. During these periods, the blower was set to move horizontally from one end of the slider to the other, while blowing towards the wall at a rate of 3.5 m3/min.
The experiments were conducted for a total of 20 days, and the chamber was closed and sealed until all experiments had been completed. Once the setup was complete, two days were allowed to pass before the experiments were initiated to ensure that all the resuspended dust due to the activities carried out for installation of the apparatus had settled down and different-sized particles’ concentration did not significantly change with time. The experiments were then initiated, and one experiment was carried out each day to ensure that the particles before the start of every experiment were in equilibrium. During these experiments, no dust was added inside the chamber, and the specific amount present inside the chamber was not known. However, knowing the exact amount of dust was not a prerequisite in this paper’s context, as this study aimed to identify how different activities would comparatively impact on the resuspension rates. Under no circumstance was anyone allowed to enter the room before the completion of all the experiments.
The particle counters were set up to start logging measurements 1 h before the start of the activation and continued until 8 h after it. The logging interval was 1 min and the logs represent the average number of particles counted every second.
Experimental outcomes
To validate the experimental data and understand the effect of different non-activated/activated protocols to air sampling fungal readings in real case scenarios, six experiments (Table 3) were conducted early in September 2022 in the living room of an apartment in east London (Fig. 5). The property was located on the third floor of a high-rise residential building and no visual signs of mould or dampness were detected anywhere inside. The occupants allowed the performance of the tests on the property and did not enter the apartment for the whole duration of the experiments to avoid affecting the sampling readings in any way.
Description of activities carried out in every test before sampling
| Experiment | Description |
|---|---|
| 1 | No activity: The apartment remained unoccupied for more than 8 h to allow any resuspended particles to settle before the commencement of the air sampling. Before initiating the sampling, the covers of the filters were removed by pulling a string from outside the living room |
| 2 | Light activity: The investigator walked inside the room for 2 min without moving objects, furniture or wiping dust off from any surface |
| 3 | Medium activity: The investigator opened the window and walked inside the room for 5 min. Any other activity was restricted |
| 4, 5 & 6 | High activity: The investigator used a leaf blower (Makita BUB182) for approx. 1 min per 10 m2 (i.e., 3 min for the approx. 30 m2 case study room), to resuspend particles from any indoor surface (the distance between the investigator and the surface being blown was instructed to be 2 m) |
-
(Source: Authors, 2022.)
The tests were designed to evaluate the effect of four different environmental settings on the sampling readings, which aimed to reflect different air-mixing (activation) states before sampling and involved carrying out what was deemed as no, light, medium and high-level activities (Table 3). The experiments for no, light and medium activity were performed once while the high activity case was repeated three times to investigate whether an activated protocol can be robust and reproducible.
The equipment was installed approximately 8.5 h before the commencement of the first experiment. Upon completion of the setup, the living room doors and windows were closed and permission to enter the room was restricted to allow resuspended particles to settle and avoid accidentally aerosolising any fungal particles from indoor surfaces. The first experiment was performed remotely using smart plugs to operate the pump and a DIY mechanism to uncover the inlet from the filters. However, while for the first experiment all activities were restricted for more than 8 h prior to sampling, the next five experiments were conducted consecutively with approximately 10 min between each and the investigator entered the space and performed the sampling manually. For all experiments the investigator wore an FFP3 mask for respiratory protection purposes.
Air sampling via filtration was used to measure indoor fungal biomass and species after different levels of activities, representative of different activated/non-activated protocols. Two mixed cellulose ester (MCE)-membrane filters (pore size 0.8 μm) provided by Mycometer A/S (Dr Neergaards Vej 3, 2970 Hørsholm, Denmark) were used for each experiment, to quantify the fungal biomass present in the sampled air. One polytetrafluoroethylene (PTFE) filter (pore size, 0.3 μm) preloaded in a 37-mm cassette was also used in every experiment to collect fungal DNA and detect the presence of 16 targeted species in the sampled air. The sampling time and volumetric flow rate were selected as 10 min and 15 l/min, respectively, for both types of filters. The filters were located within holes, cut based on the filter dimensions and at 75 cm from each other, through a 5-mm thick acrylic panel, fixed on top of a tripod such that the sampling is done at a height of around 1.3 m. This panel was built by the researcher in an effort to conduct both β-N-acetylhexosaminidase (NAHA) and DNA sampling simultaneously. An SKC BioLite+ (Blandford Forum, UK) with adjustable backpressure that allowed flow calibration was used for the DNA sampling, while a pump provided by Mycometer A/S was used for the NAHA testing. The tripod was located in the middle of the room for sampling. Upon completion of the tests, the MCE-membrane and PTFE filters were stored for 1 day at room temperature and in a −80°C freezer in-house and were sent to Mycometer A/S and HouseTest ApS (Petersmindevej 1A, 5000 Odense, Denmark) 1 and 2 days after the sampling, respectively.
The quantification of the fungal biomass was performed by Mycometer A/S using fluorogenic detection of different hydrolase activities (NAHA; EC 3.2.1.52). Hydrolase substrate containing a fluorophore was contacted with the membrane MCE filters. The enzyme activity was determined by measuring the amount of fluorescence formed during the reaction (following the protocol of the manufacturer, Mycometer A/S). The fluorescence signals from the fungi and the total allergens were then extracted and reported in relative fluorescence units (RFU), along with the Fungal to Allergen Index (FAI) (ratio of the fungi to allergen levels).
Species identification was performed by HouseTest ApS through the extraction and amplification of DNA from the PTFE filters. Quantitative polymerase chain reaction (qPCR) analysis was performed using primers targeting the DNA of (a) 16 fungal species (Acremonium strictum, Alternaria alternata, A. fumigatus, A. versicolor, A. niger, Chaetomium globosum, C. cladosporioides, C. herbarum, C. sphaerospermum, P. chrysogenum, P. expansum, Rhizopus stolonifer, Stachybotrys chartarum, Trichoderma viride, Ulocladium chartarum, Wallemia sebi), and (b) three fungal groups (Aspergillus glaucus grp., Mucor/Rhizopus grp., Penicillium/Aspergillus/Paecilomyces variotii grp.). The internal transcribed spacer (ITS) region of the nuclear ribosomal repeat unit was targeted by the primer sequences used as described by Lu et al. [45].
Results
Laboratory experiments
The outcomes of the experimental work are shown in Fig. 6. The results indicate that the particle resuspension rates increase with the increase of the blowing time for all three sets of experiments, with up to X and Y times difference for small (particle matter [PM]a-b) and large particles (PMc-d), respectively, between sampling in still conditions and after 15 min of activation. Importantly, for blowing durations of more than 5 min, the smaller particles (particle size <PM1) did not return to the levels before the blowing was initiated, even after 7 h (Fig. 6a–c). However, the same cannot be stated for larger particles – for the same blowing duration, the pattern of the particle settlement becomes more unclear with the increase in the aerodynamic diameter (AD) (Fig. 6d–f).
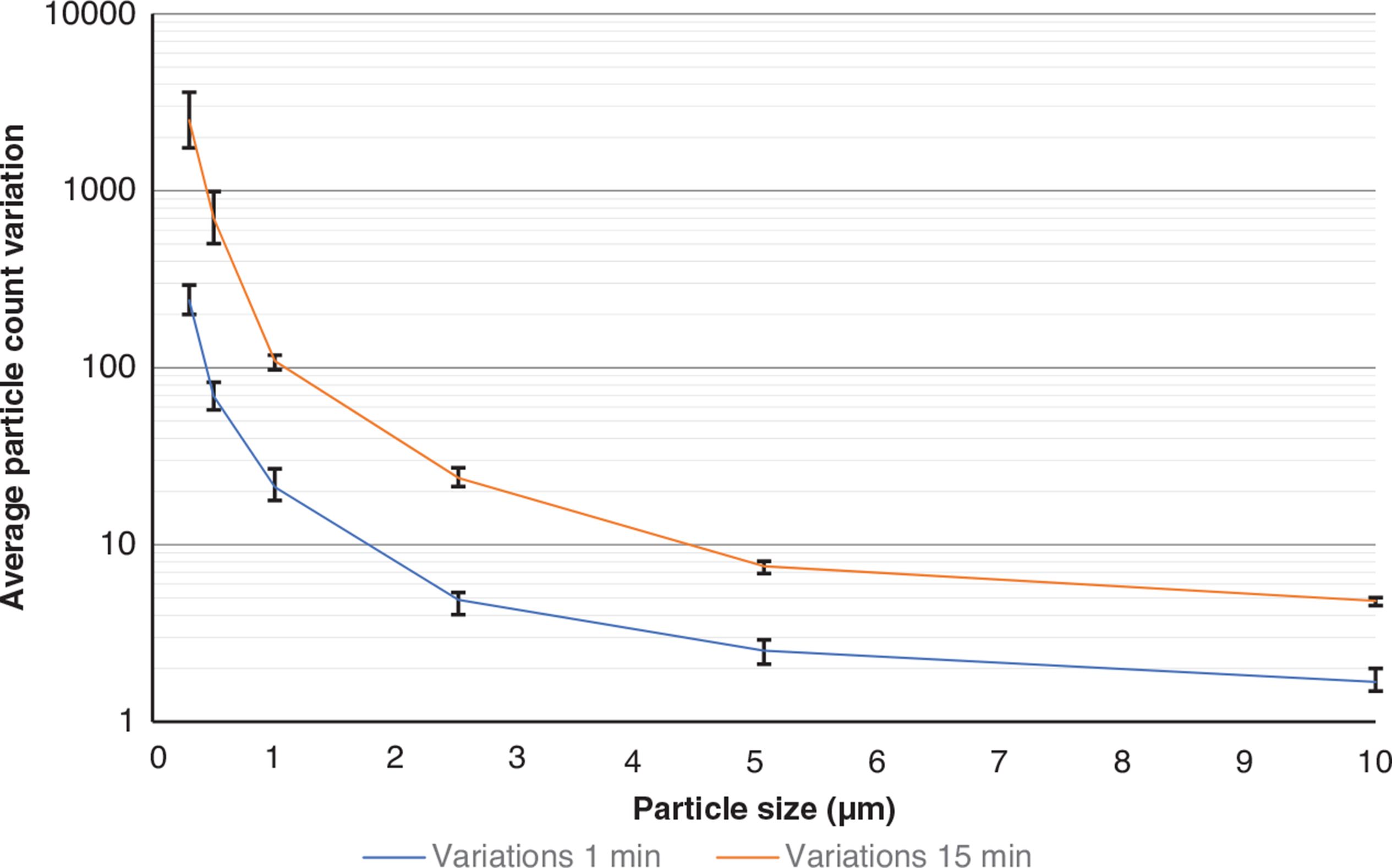
Particle counts for (a) PM0.3, (b) PM0.5, (c) PM1.0, (d) PM2.5, (e) PM5.0 and (f) PM10. Different segments in each plot delineated in different colours indicate three repeated sets of each test. The findings are indicated for 1, 2, 3, 5, 10 and 15 min of blowing and for each of the three series as shown in (a). (Source: Authors, 2022.)
To better understand the pattern of the particle readings with the increase of the blowing duration, the average number of the PM0.3, PM2.5 and PM10 over 1-min intervals were plotted against time for three cases of blowing duration: 1 min, 5 min and 15 min (Fig. 7). As seen, higher blowing durations led to higher resuspension rates for all three size particles at any height.
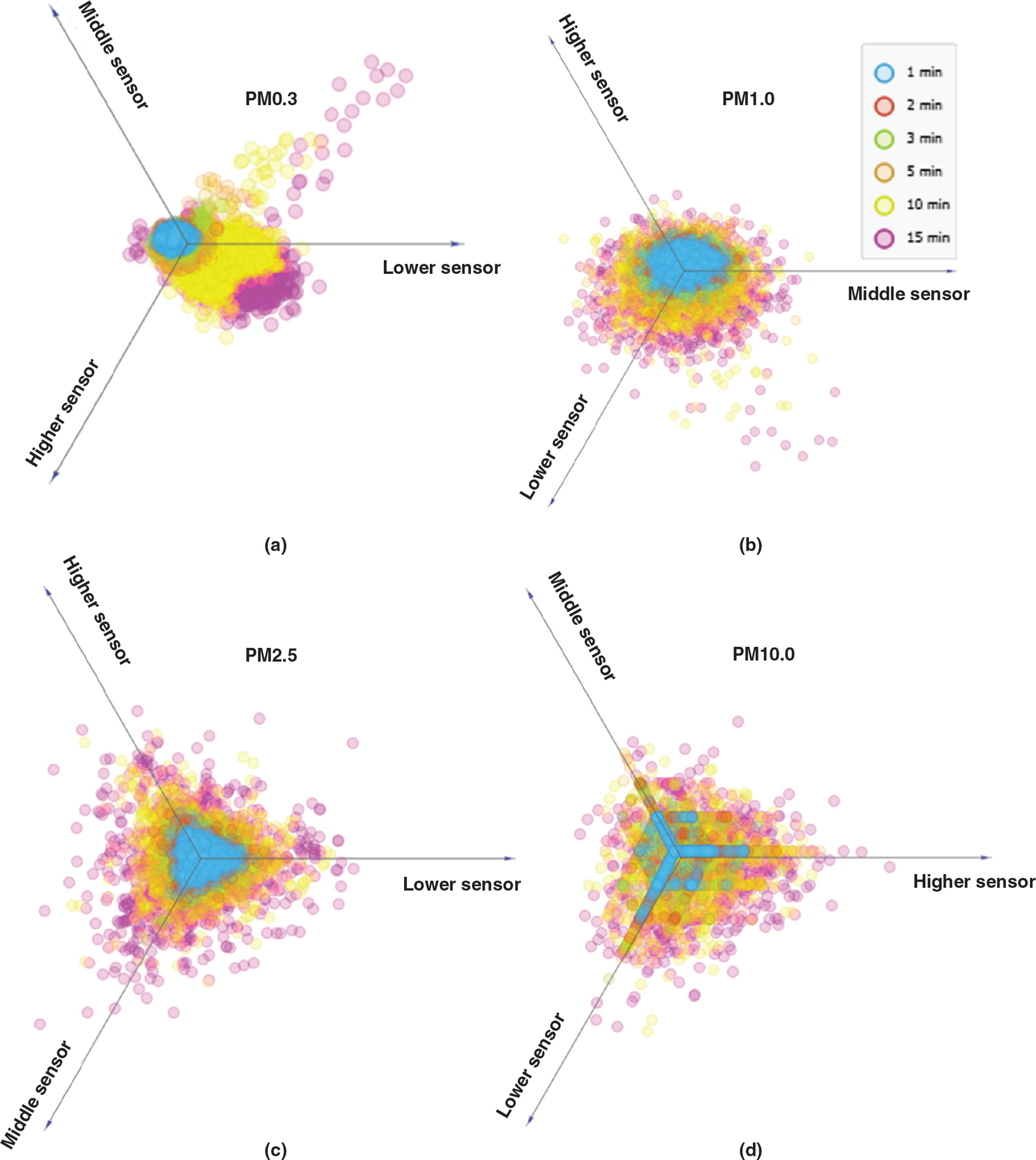
The number of different-sized particles measured at different heights was linearly projected to further study the effect of the blowing duration on the particle counts with height (Fig. 8). The increase in the blowing duration affected the resuspension rate of both small and large particles. The difference in the particle numbers due to the change in the blowing duration is better captured by the lower particle sensor than the middle and higher one (Fig. 8a). However, Fig. 8c–d shows a more uniform increase in the levels of the particles when their AD is larger than 1 μm with increasing blowing durations compared to the levels of PM0.3 and PM0.5 (Fig. 8a–b). Higher levels of the small particles (PM0.3 and PM0.5) were measured by the lower sensor than the higher ones.
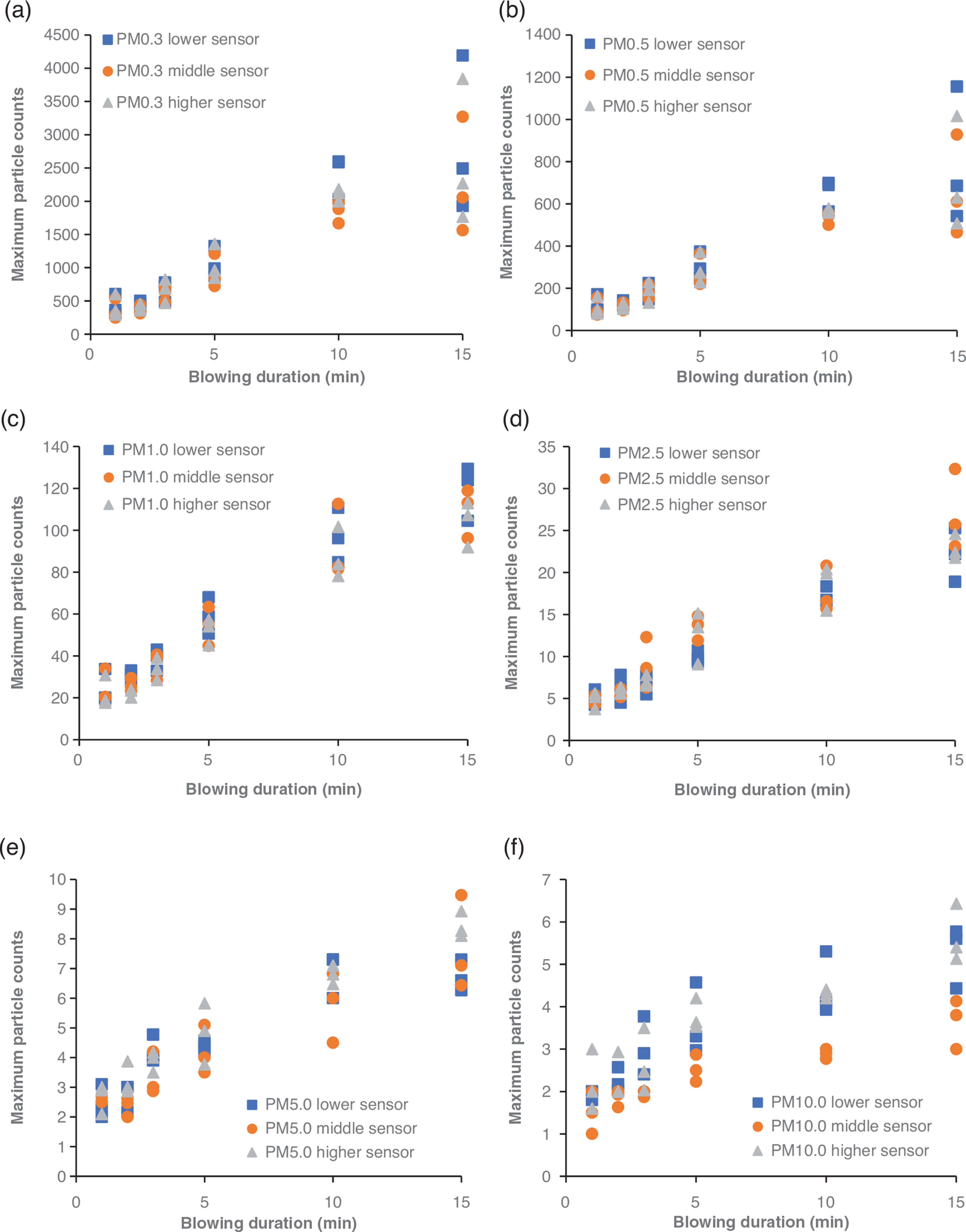
The maximum number of particles measured at each height in every experiment was plotted against the blowing duration and is demonstrated in Fig. 9a–f. The coefficients of determination [R2] for all three series suggest that the maximum number of particles of all sizes is strongly correlated to the blowing duration with the PM1.0 and PM2.5. In conjunction with Figs 6 and 7, the increasing trend of the maximum values of particles with the prolongation of the blowing duration suggests that the increase of the activity duration prior to sampling leads to higher particle resuspension rates.
Pearson’s correlation coefficient was calculated to identify potential correlations between the number of different-sized particles and the height, and a correlation matrix was created (Table 4). The results of the analysis indicate that the small particles (PM0.3–PM2.5) correlate strongly with each other regardless of the height of the sensor (r > 0.6). On the other hand, no strong correlations between the larger particles (PM5 and PM10) were identified with the highest value of r being 0.215 for PM5 particles measured by the higher and lower sensor and the lower R-value being the one for PM10 particles measured by the lower and higher sensor (r = 0.058). It should be noted that in a previous study by Luoma and Batterman [46], no significant variation was reported between readings of PM1 or smaller particles with height (particle counters placed at 0.4, 1.1, 1.8 m). On the other hand, the readings of particles ranging from PM5.0 to PM25 were shown to vary significantly with height [42].
Correlation coefficient for same-sized particles measured at different heights
| PM0.3 low sensor | PM0.3 high sensor | ||
|---|---|---|---|
| PM0.3 low sensor | 1.000 | ||
| PM0.3 mid sensor | 0.993 | 1.000 | |
| PM0.3 high sensor | 0.986 | 0.986 | 1.000 |
| PM0.5 low sensor | PM0.5 mid sensor | PM0.5 high sensor | |
| PM0.5 low sensor | 1.000 | ||
| PM0.5 mid sensor | 0.993 | 1.000 | |
| PM0.5 high sensor | 0.985 | 0.986 | 1.000 |
| PM1.0 low sensor | PM1.0 mid sensor | PM1.0 high sensor | |
| PM1.0 low sensor | 1.000 | ||
| PM1.0 mid sensor | 0.964 | 1.000 | |
| PM1.0 high sensor | 0.951 | 0.953 | 1.000 |
| PM2.5 low sensor | PM2.5 mid sensor | PM2.5 high sensor | |
| PM2.5 low sensor | 1.000 | ||
| PM2.5 mid sensor | 0.606 | 1.000 | |
| PM2.5 high sensor | 0.606 | 0.611 | 1.000 |
| PM5.0 low sensor | PM5.0 mid sensor | PM5.0 high sensor | |
| PM5.0 low sensor | 1.000 | ||
| PM5.0 mid sensor | 0.142 | 1.000 | |
| PM5.0 high sensor | 0.215 | 0.211 | 1.000 |
| PM10 low sensor | PM10 mid sensor | PM10 high sensor | |
| PM10 low sensor | 1.000 | ||
| PM10 mid sensor | 0.096 | 1.000 | |
| PM10 high sensor | 0.058 | 0.090 | 1.000 |
-
(Source: Authors, 2022.)
The Levene test was used to examine the equality of the variance for the number of same-sized particles measured by the same sensor during all three experiments. For a significance level of P value = 0.05, the tests have indicated no homogeneity of the variances between the three experimental series for all cases tested. This suggests that although the tests were repeated in the exact same way the variances of the readings are significantly different. These differences might be a result of the limited horizontal movement of the blower and the limited area being directly affected by the high air velocity output. Nevertheless, this conforms with the literature outcomes in the Materials and methods section and adds extra value to the statement regarding the comparability concerns due to limited control over the activities and the testing conditions prior to sampling.
Furthermore, to understand the extent of the activation’s effect on the particle readings, the differences between the maximum and minimum particle counts captured by every sensor were averaged for all experiments carried out for 1 min and 15 min blowing durations and plotted against particle size in Fig. 10. The blowing duration has led to an increase in the particle count variation for all particle sizes – with the difference being the largest for the smaller particles (>4000 particles for PM0.3 and 15 min blowing) and PM10 particles (<7 particles) and blowing duration of 15 min. This can indicate that the readings for the PM1.0 or smaller particles capture the blowing duration changes more easily than the larger particle counts. Therefore, monitoring the small-sized particles (<PM1.0) could be a better proxy of the intensity of the activities carried out prior to sampling. It is important to mention that despite the prolonged blowing duration (15 min), the variation in the large-particle readings (>PM2.5) was only slightly larger than the corresponding one when the blowing duration was 1 min, indicating that the air activation might be more critical for the recovery efficiency of PM2.5 and larger particles during the sampling.
Case study outcomes
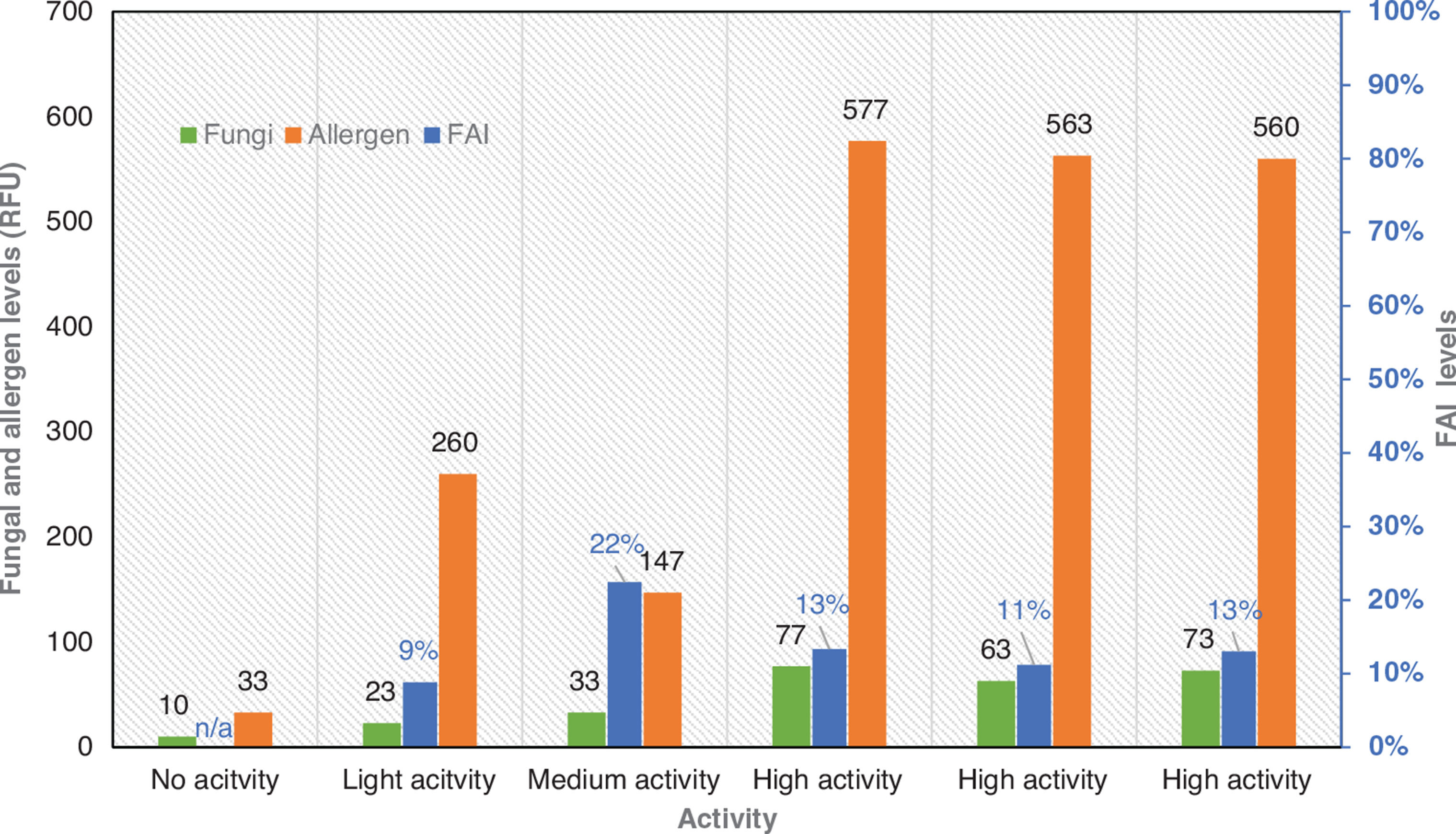
The Mycometer A/S method enabled the extraction of information with regard to three fungal growth indicators.
-
The fungal levels: Shows how many fungal particles have been detected in the first filter used during sampling.
-
The allergen levels: Shows the number of total allergens (dust mites, pollen, fungi, pet dander, skin cells, etc.) identified on the second filter used during sampling.
-
FAI: Measures the ratio between the two previous markers.
The fungal and allergen levels measured in the case study room indicated that the intensity of the activities prior to sampling has a noticeable impact on the testing readings (Fig. 11). The first experiment (no activity) allowed the detection of very small concentrations of fungi (10 RFU) and allergens (33 RFU), while the results from the high activity experiments led to the capture of approximately seven and 17 times more fungal and allergen particles in the MCE filters, respectively. It should be underlined that although the fungal levels increased when the activities intensified, the allergen levels did not follow the same trend. The allergen readings from the medium activity experiment were found to be lower than the corresponding ones from the light activity experiment. The underestimation of the allergen levels in the tested space might have been a result of the opening of the windows and the air exchange between a potentially poorer-in-allergens outdoor environment and an indoor environment with higher allergen levels. It is also worth noticing that the FAI indicators did not follow a particular trend with the increase of the activities’ intensity. However, while the FAI ratios deviated noticeably in the first three experiments (no, light and medium activity), the deviation between the high activity ratios was rather minimal.
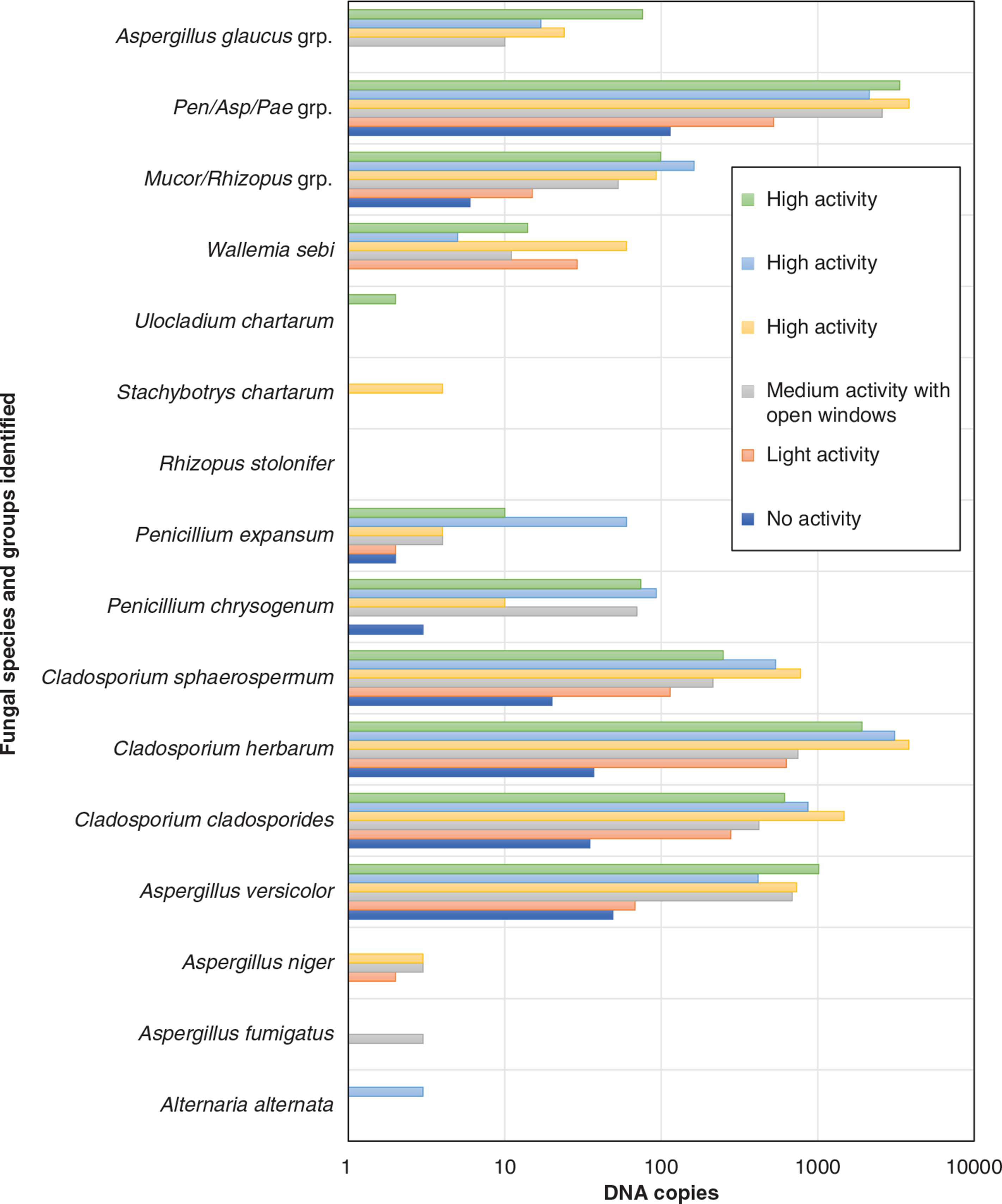
Out of the 16 species that were targeted via the DNA amplification only six species were detected when activities prior to sampling were restricted completely (Fig. 12). However, with the increase of the activity’s intensity, the number of identified species rose to seven for light activity, 10 for medium activity, and nine, nine and 10 for high activity experiments. Although the number of DNA clones for every group or species identified varied with the increase of the activity intensity, the fact that the samples from the no and light activity tests have captured two to three species less than the medium and high activity tests might mean that non-activated protocols might be underestimating fungal activity in the rooms. In addition, A. fumigatus was only detected in a very small concentration in the sample from the medium activity test where the windows were open – this species that is known to live in compost and garbage outside [47] is likely to have appeared in the sample due to the air exchange between the indoor and outdoor environment. That Uladicladium chartarum and Stachbotryn chartarum appearing only under full activation conditions is also very telling, as they have larger spores than the others (around 8–12 × 4–5 μm and up to 20 × 16 μm, respectively).
Discussion
The laboratory experiment has shown that the particle readings, regardless of the particle size, follow an increasing trend with the increase of the blowing duration. However, the impact of air activation on larger particle readings (>PM2.5) was considerably weaker than the effect of the smaller ones (<PM2.5). Previous studies [31,48] have shown that the indoor air velocity close to a dust reservoir and the activation method used can affect the particle resuspension rates. Still, they have also stressed that large particles might be more inert to activation due to their size and the strong adhesion bonds between them and the surfaces they are attached to. Therefore, investigators should consider that a strict non-activated protocol might seriously underestimate the particle intensity with important implications in relation to the indoor fungal levels and the health impact.
The particle counters’ low sensitivity for PM0.3 readings (50% sensitivity) indicates that the readings of this channel might not be an accurate proxy for the evaluation of the indoor activities before sampling. Still, they might be able to show a trend for the PM0.3 concentration. Given also that the majority of fungal particles will range between 1.0 μm AD and 3.2 μm AD [42], it can be stated that the PM0.3 readings would not be a suitable metric for the indoor fungal resuspension rates. On the other hand, the high sensitivity (>98%) of the sensor for particles larger than PM0.5 and the considerably higher particle count differences for smaller-sized particles (<PM1.0) with the increase of the blowing duration (Fig. 10) indicates that the PM0.5 and PM1.0 readings reported here could be the most appropriate indicators of the activities carried out before sampling.
The high correlation coefficients between readings obtained at different heights for <PM2.5 (Table 4) indicate that the height at which the sensors are placed will not significantly affect their ability to capture the concentration changes of particles. This conforms with previous studies showing that regardless of the sensor’s height, unlike the coarser particles (>PM5.0), the smaller airborne particle counts will follow similar trends due to their ability to spread throughout an indoor space unrestricted of their size [49]. However, the same cannot be stated for larger particles (>PM2.5) where the low correlation coefficients suggest that the height of the sensor had led to noticeable differences in the particle count changes with time.
When looking at the case study outcomes, the small deviations between the FAI, fungi and allergen levels from the high activity tests, especially when compared to the deviations between the other cases, suggest that activated protocols, if well-defined, are reproducible and provide robust fungal measurements. The results indicate that the increase of the activity intensity prior to sampling affects the fungal readings. The increase of the fungal readings with the escalation of the activities, comes in agreement with previous studies by Rylander [26] and Aktas et al. [15], who showed that the use of a blower prior to sampling led to an up to 10 and 56 times increase in the testing outcomes compared to the no and low activity readings, respectively. Airing the room before the sampling, such as through opening the windows, might lead to measurements that may not be representative of the indoor fungal activity due to the mixing of indoor and outdoor air, as shown by the medium activity testing in our case study.
The intensification of activities prior to sampling has allowed the detection of more species than the ones captured from the no activity case. However, while the low activity experiment allowed the identification of an additional species in the sample all other experiments led to the detection of three or more species. This is in broad agreement with the hypothesis that air activation can allow the aerosolisation and detection of fungal particles that develop stronger bonding forces with the substrate materials and would not be identified via non-activated protocols carried out prior to sampling. It is also noteworthy that species with large spores were not captured by tests done following a no-, low- or medium-level of activity – an observation in line with the experimental outcome that the large-size particles are harder to suspend and hence to sample.
Conclusions
Non-activated protocols, currently dominating the literature, can heavily underestimate the fungal biomass and the species present within a given indoor space, with serious implications for studies focussed on health and building condition alike. Researchers should give attention to the conditions under which indoor fungal testing is carried out. Outcomes of non-activated and activated testing do differ, therefore indoor fungal levels cannot be evaluated or benchmarked unless uniformity is brought to the pre-sampling conditions through a well-established testing protocol. Disturbing the air’s stillness through activation does increase the concentration of captured airborne fungal fragments and spores, and thus leads to the detection of fungi that could otherwise be undetectable. The experimental work carried out here suggests that the use of a blower and the increase of the blowing duration lead to higher particle resuspension. In real-case scenarios this manifests as higher detectability of particles ranging from 0.3 to 10 μm, as supported also by the case study testing, we present here. The findings suggest that the smaller particle (PM0.3 to PM1.0) readings are more responsive to the different levels of activity than the larger particles (PM2.5 and PM5.0). The sensitivity of the testing outcomes to the level of activity within the testing space prior to sampling also suggests that the non-activated protocols, if chosen, must control very closely the use of the room for several hours prior to the sampling. In practice this may be very difficult, but an inability to control the conditions prior to sampling can lead to outcomes that are not comparable with other studies.
Therefore, the selection of an activated protocol is of critical importance for collection efficiency, both in terms of fungal biomass and the number of identified species. As shown by the repeated testing in our case study, a blowing duration of 1 min/10 m2 proves to be efficient to capture the fungal activity and leads to stable readings. Furthermore, the experimental study presented here suggests that the sampling height is another testing protocol variable that the larger particles are more sensitive to and should be paid attention to for comparability purposes. Our work shows that while any height between 0.75 and 1.5 m should work well, sampling at higher elevations might not be able to capture larger size particles. Furthermore, activities that could lead to an increase/decrease of contaminants indoors, such as the opening of windows, can have unclear effects on the fungal measurements and should be avoided.
Acknowledgements
This study has been carried out as part of the EPSRC-DTP-CASE (EP/R513143/1) project where POLYGON UK was the industry sponsor. The authors would like to acknowledge that the funders had no role in the study design, data collection and interpretation or the decision to submit the work for publication. We are greatly thankful to both funding bodies.
We would also like to thank Mycometer A/S and Housetest ApS for their significant in-kind contribution to our work via the performance of the quantitative analysis and species identification respectively.
We’d like to extend our gratitude to Dr Birgitte Andersen and Dr Jonathon Taylor for their valuable comments which immensely helped improve our paper and make it to its current state.
Open data and materials availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations and conflicts of interest
Research ethics statement
The authors declare that research ethics approval for this article was provided by the UCL Research Ethics Committee (Ethics ID: 20767/001 - UCL Data protection officer: Alex Potts).
Consent for publication statement
The authors declare that research participants’ informed consent to publication of findings – including photos, videos and any personal or identifiable information – was secured prior to publication.
Conflicts of interest statement
The authors declare no conflicts of interest with this work.
References
[1] World Health Organization Europe. WHO guidelines for indoor air quality: dampness and mould, : 1–228.
[2] World Health Organization Europe. Sarigiannis, DA (ed.), . (2013). Combined or Multiple Exposure to Health Stressors in Indoor Built Environments, Bonn, Germany:
[3] Andersson, MA; Nikulin, M; Köljalg, U; Andersson, MC; Rainey, F; Reijula, K. (1997). Bacteria, molds, and toxins in water-damaged building materials. Appl Environ Microbiol 63 (2) : 387–393, DOI: http://dx.doi.org/10.1128/aem.63.2.387-393.1997
[4] Singh, J. (1999). Review: dry rot and other wood-destroying fungi: their occurrence, biology, pathology and control. Indoor Built Environ 8 (1) : 3–20, DOI: http://dx.doi.org/10.1177/1420326X9900800102
[5] Heinsohn, PA. (2007). Conducting building mold investigations In: Yang, CS, Heinsohn, PA PA (eds.), Sampling and Analysis of Indoor Microorganisms. Pacifica, CA: Wiley Interscience.
[6] BSI. BS ISO 16000-19: 2014 Indoor Air: Sampling strategy for moulds. Brussels: CEN.
[7] Aktas, YD; Altamirano, H; Ioannou, I; May, N; D’Ayala, DF. (2018). Indoor Mould Testing and Benchmarking: Public Report. February 2018 : 1–16.
[8] Aktas, YD; Reeslev, M; Altamirano, H; May, N; D’Ayala, DF. (2020). Normal background levels of air and surface mould reserve in English residential building stock: a preliminary study towards benchmarks based on NAHA measurements. UCL Open Environment 2 (1) DOI: http://dx.doi.org/10.14324/111.444/ucloe.000005
[9] Terčelj, M; Salobir, B; Harlander, M; Rylander, R. (2011). Fungal exposure in homes of patients with sarcoidosis – an environmental exposure study. Environ Health 10 (1) 8 DOI: http://dx.doi.org/10.1186/1476-069X-10-8
[10] Shinohara, N; Woo, C; Yamamoto, N; Hashimoto, K; Yoshida-Ohuchi, H; Kawakami, Y. (2021). Comparison of DNA sequencing and morphological identification techniques to characterize environmental fungal communities. Sci Rep 11 : 2633. DOI: http://dx.doi.org/10.1038/s41598-021-81996-w
[11] Canha, N; Almeida, SM; Freitas, MC; Wolterbeek, HT. (2015). Assessment of bioaerosols in urban and rural primary schools using passive and active sampling methodologies. Arch Environ Prot 41 (4) : 11–22, DOI: http://dx.doi.org/10.1515/aep-2015-0034
[12] Gent, JF; Kezik, JM; Hill, ME; Tsai, E; Li, D-W; Leaderer, BP. (2012). Household mold and dust allergens: exposure, sensitization and childhood asthma morbidity. Environ Res 118 : 86–93, DOI: http://dx.doi.org/10.1016/j.envres.2012.07.005
[13] Dallongeville, A; Cann, PL; Zmirou-Navier, D; Chevrier, C; Costet, N; Annesi-Maesano, I. (2015). Concentration and determinants of molds and allergens in indoor air and house dust of French dwellings. Sci Total Environ 536 : 964–972, DOI: http://dx.doi.org/10.1016/j.scitotenv.2015.06.039
[14] Nieto-Caballero, M; Gomez, OM; Shaughnessy, R; Hernandez, M. (2022). Aerosol fluorescence, airborne hexosaminidase, and quantitative genomics distinguish reductions in airborne fungal loads following major school renovations. Indoor Air 32 (1) DOI: http://dx.doi.org/10.1111/ina.12975
[15] Aktas, YD; Ioannou, I; Altamirano, H; Reeslev, M; D’Ayala, D; May, N. (2018). Surface and passive/active air mould sampling: a testing exercise in a north London housing estate. Sci Total Environ 643 : 1631–1643, DOI: http://dx.doi.org/10.1016/j.scitotenv.2018.06.311
[16] Aktaş, YD; Shi, J; Blades, N; D’Ayala, D. (2018). Indoor mould testing in a historic building: Blickling Hall. Herit Sci 6 (51) : 1–9, DOI: http://dx.doi.org/10.1186/s40494-018-0218-x
[17] Buttner, MP; Stetzenbach, LD. (1993). Monitoring airborne fungal spores in an experimental indoor environment to evaluate sampling methods and the effects of human activity on air sampling. Appl Environ Microbiol 59 : 219–226.
[18] Miller, JD. (2011). Mycological investigations of indoor environments In: Flannigan, B, Samson, RA; RA and Miller, JD JD (eds.), Microorganisms in Home and Indoor Work Environments: Diversity, Health Impacts, Investigation and Control. 2nd ed Boca Raton: CRC Press.
[19] Rao, CY; Burge, HA; Chang, JCS. (1996). Review of quantitative standards and guidelines for fungi in indoor air. J Air Waste Manage Assoc 46 (9) : 899–908, DOI: http://dx.doi.org/10.1080/10473289.1996.10467526
[20] Efthymiopoulos, S; Aktas, Y; Altamirano, H. (2021). Air sampling and analysis of indoor fungi: a critical review of passive (non-activated) and active (activated) sampling In: 1st International Conference on Moisture in Buildings 2021. ICMB21. DOI: http://dx.doi.org/10.14293/ICMB210021
[21] Swaebly, MA; Christensen, CM. (1952). Molds in house dust, furniture stuffing, and in the air within homes. J Allergy 23 (4) : 370–374, DOI: http://dx.doi.org/10.1016/0021-8707(52)90058-0
[22] Grinshpun, SA. (2010). Biological aerosols In: Agranovski, I (ed.), Aerosols – Science and Technology. Weinheim: Wiley-VCH.
[23] Amato, P; Brisebois, E; Draghi, M; Duchaine, C; Fröhlich-Nowoisky, J; Huffman, JA. (2018). Sampling techniques In: Delort, A-M, Amato, P P (eds.), Microbiology of Aerosols. Hoboken, NJ: John Wiley & Sons.
[24] Mainelis, G. (2020). Bioaerosol sampling: classical approaches, advances, and perspectives. Aerosol Sci Technol 54 : 496–519, DOI: http://dx.doi.org/10.1080/02786826.2019.1671950
[25] Flannigan, B. (1992). Indoor microbiological pollutants – sources, species, characterisation and evaluation In: Knoppel, H, Wolkoff, P P (eds.), Chemical, Microbiological, Health and Comfort Aspects of Indoor Air Quality Gate of the Art in SBS. Dordrecht: Kluwer, p. 73398.
[26] Rylander, R. (2015). β-N-Acetylhexosaminidase (NAHA) as a marker of fungal cell biomass – storage stability and relation to β-glucan. Int J Environ Monit Anal 3 (4) : 205. DOI: http://dx.doi.org/10.11648/j.ijema.20150304.11
[27] Mundt, E. (2000). Particles and displacement ventilation In: Awbi, H (ed.), Air Distribution in Rooms (ROOMVENT 2000). Reading: Elsevier Science, pp. 737–742.
[28] Mukai, C; Siegel, JA; Novoselac, A. (2009). Impact of airflow characteristics on particle resuspension from indoor surfaces. Aerosol Sci Technol 43 (10) : 1022–1032, DOI: http://dx.doi.org/10.1080/02786820903131073
[29] Goldasteh, I; Ahmadi, G; Ferro, A. (2010). Effect of air flow on dust particles resuspension from common flooring In: ASME 2010 3rd Joint US–European Fluids Engineering Summer Meeting: Volume 1, Symposia – Parts A, B, and C. ASME 2010 3rd Joint US-European Fluids Engineering Summer Meeting collocated with 8th International Conference on Nanochannels, Microchannels, and Minichannels. Montreal, Quebec, Canada ASMEDC, pp. 2797–2800, DOI: http://dx.doi.org/10.1115/FEDSM-ICNMM2010-30596
[30] Napoli, C; Marcotrigiano, V; Montagna, MT. (2012). Air sampling procedures to evaluate microbial contamination: a comparison between active and passive methods in operating theatres. BMC Public Health 12 (1) 594 DOI: http://dx.doi.org/10.1186/1471-2458-12-594
[31] Wang, B; Tang, Z; Li, Y; Cai, N; Hu, XX. (2021). Experiments and simulations of human walking-induced particulate matter resuspension in indoor environments. J Clean Prod 295 126488 DOI: http://dx.doi.org/10.1016/j.jclepro.2021.126488
[32] Zorman, T; Jeršek, B. (2008). Assessment of bioaerosol concentrations in different indoor environments. Indoor Built Environ 17 (2) : 155–163, DOI: http://dx.doi.org/10.1177/1420326X08089251
[33] Adams, RI; Miletto, M; Taylor, JW; Bruns, TD. (2013). Dispersal in microbes: fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J 7 (7) : 1262–1273, DOI: http://dx.doi.org/10.1038/ismej.2013.28
[34] Cao, S-J; Cen, D; Zhang, W; Feng, Z. (2017). Study on the impacts of human walking on indoor particles dispersion using momentum theory method. Build Environ 126 : 195–206, DOI: http://dx.doi.org/10.1016/j.buildenv.2017.10.001
[35] Metz, B; Kossen, NWF; Suijdam, JC. (1979). The rheology of mould suspensions. Adv Biochem Engin/Biotechnol 11 : 103–156, DOI: http://dx.doi.org/10.1007/3-540-08990-X_24
[36] Tucker, K; Stolze, JL; Kennedy, AH; Money, NP. (2007). Biomechanics of conidial dispersal in the toxic mold Stachybotrys chartarum. Fungal Genet Biol 44 (7) : 641–647, DOI: http://dx.doi.org/10.1016/j.fgb.2006.12.007
[37] Arthurs, S; Thomas, MB. (2001). Effects of temperature and relative humidity on sporulation of Metarhizium anisopliae var. acridum in mycosed cadavers of Schistocerca gregaria . J Invertebr Pathol 78 (2) : 59–65, DOI: http://dx.doi.org/10.1006/jipa.2001.5050
[38] Zhao, S; Shamoun, SF. (2006). The effects of culture media, solid substrates, and relative humidity on growth, sporulation and conidial discharge of Valdensinia heterodoxa . Mycol Res 110 (11) : 1340–1346, DOI: http://dx.doi.org/10.1016/j.mycres.2006.08.001
[39] Money, NP. (2016). Spore production, discharge, and dispersal In: Watkinson, SC, Boddy, L; L and Money, NP NP (eds.), The Fungi. Waltham, MA: Academic Press, pp. 67–97, DOI: http://dx.doi.org/10.1016/B978-0-12-382034-1.00003-7
[40] Osherov, N; May, GS. (2001). The molecular mechanisms of conidial germination. FEMS Microbiol Lett 199 (2) : 153–160, DOI: http://dx.doi.org/10.1111/j.1574-6968.2001.tb10667.x
[41] Baron, PA; Kulkarni, P; Willeke, K. (2011). Aerosol Measurement: Principles, Techniques, and Applications. 3rd ed Hoboken, NJ: Wiley.
[42] Clauß, M. (2015). Particle size distribution of airborne microorganisms in the environment – a review. Landbauforschung - Appl Agric Forestry Res (2/2015) : 77–100, DOI: http://dx.doi.org/10.3220/LBF1444216736000
[43] Agranovski, V; Ristovski, Z; Blackall, PJ; Morawska, L. (2004). Size-selective assessment of airborne particles in swine confinement building with the UVAPS. Atmos Environ 38 (23) : 3893–3901, DOI: http://dx.doi.org/10.1016/j.atmosenv.2004.02.058
[44] Brandl, H; von Däniken, A; Hitz, C; Krebs, W. (2008). Short-term dynamic patterns of bioaerosol generation and displacement in an indoor environment. Aerobiologia 24 (4) : 203–209, DOI: http://dx.doi.org/10.1007/s10453-008-9099-x
[45] Lu, R; Pørneki, AD; Lindgreen, JN; Li, Y; Madsen, AM. (2021). Species of fungi and pollen in the PM1 and the inhalable fraction of indoor air in homes. Atmosphere 12 (3) 404 DOI: http://dx.doi.org/10.3390/atmos12030404
[46] Luoma, M; Batterman, SA. (2001). Characterization of particulate emissions from occupant activities in offices. Indoor Air 11 (1) : 35–48, DOI: http://dx.doi.org/10.1034/j.1600-0668.2001.011001035.x
[47] Samson, RA; Houbraken, J; Thrane, U; Frisvad, JC; Andersen, B. (2019). Food and Indoor Fungi. Centraalbureau voor Schimmelcultures.
[48] Qian, J; Ferro, AR. (2008). Resuspension of dust particles in a chamber and associated environmental factors. Aerosol Sci Technol 42 (7) : 566–578, DOI: http://dx.doi.org/10.1080/02786820802220274
[49] Montoya, LD; Hildemann, LM. (2005). Size distributions and height variations of airborne particulate matter and cat allergen indoors immediately following dust-disturbing activities. J Aerosol Sci 36 (5–6) : 735–749, DOI: http://dx.doi.org/10.1016/j.jaerosci.2004.11.004