Introduction
Larger benthic foraminifera (LBF) are single-celled organisms, with an internal, characteristic test, that have acted as major reef-forming organisms from the Paleozoic to the present day (see [ 1]). Within the geological record, there are 14 taxonomically defined orders of LBF. They are widely used as key biostratigraphic and palaeoenvironmental indicators. Although they were generally limited to warmer, shallower marine environments, their fossil forms are globally distributed, but frequently exhibit provincialism at the family or generic level.
Our previous work has established that global marine regressions correlate with the migration of some LBF genera from one province to another. The inter-provincial migration of a genus is characterised and defined by the contemporaneous occurrence of the same species (although they may have been given different names, which are therefore synonyms) in two previously isolated provinces. In the Cretaceous, for example, we inferred that, during a global sea-level low stand, some orbitolinids (agglutinated foraminifera, members of the Order Textulariida) underwent a westward trans-oceanic migration from their Tethyan province of origin to the American province (see [ 2]). Although subsequently the Tethyan province remained the hotspot for orbitolinid speciation, parallel lineages evolved in the again isolated American province, but were extremely rare. Later in the Cenozoic, the American province became the hot spot for speciation of other LBF families (including members of the Order Rotaliida, studied previously by BouDagher-Fadel and Price [ 3– 6]). Again during global sea-level regressions, trans-Atlantic migrations occurred, but in these cases in an eastward direction into Tethys and then on to the Indo-Pacific, and at times to southern Africa.
These previous studies, therefore, have led to the hypothesis that periodic, sea-level low stand enabled, inter-provincial migration is a characteristic global dispersal mechanism of many LBF families. As suggested by Pignatti [ 7] however, this hypothesis needs further testing. In this study, therefore, we investigate the phylogenetic evolution and palaeogeographic distribution of the main genera of the porcelaneous Alveolinoidea superfamily (members of the Order Miliolida), to establish whether they too exhibit an evolutionary and dispersal behaviour similar to the previously studied LBF groups.
The Alveolinoidea comprise three families, the Alveolinidae, the Fabulariidae and the Rhapydioninidae (see [ 1]). They range from the Cretaceous to the present day. Throughout their evolutionary lineages, the alveolinoids exhibited relatively rapid rates of evolution and developed complex tests, which make them an important biostratigraphic index fossil group for the shallow-marine environments of the later Mesozoic and the Cenozoic. Today, alveolinoid-bearing limestones occur globally from the Americas, through the Mediterranean and the Gulf, Tibet, and on to the Indo-Pacific province. They are often associated with significant hydrocarbon reservoirs. The study of the Alveolinoidea and the definition of their stratigraphic ranges has been the subject of many regional micropaleontological investigations, especially those of the Tethyan and American provinces [ 1, 8– 22], but the most recent revisions of Alveolina from Indonesia are by Bakx [ 23]. Until now, however, it has not been possible to develop an effective global view of their evolution and inter-relationships, because the systematic study of the links between the American, Tethys and Indo-Pacific lineages has been hampered by the lack of biostratigraphic correlation between the geographically scattered alveolinoid assemblages described in the literature, and by the scarcity of described material from the Indo-Pacific province.
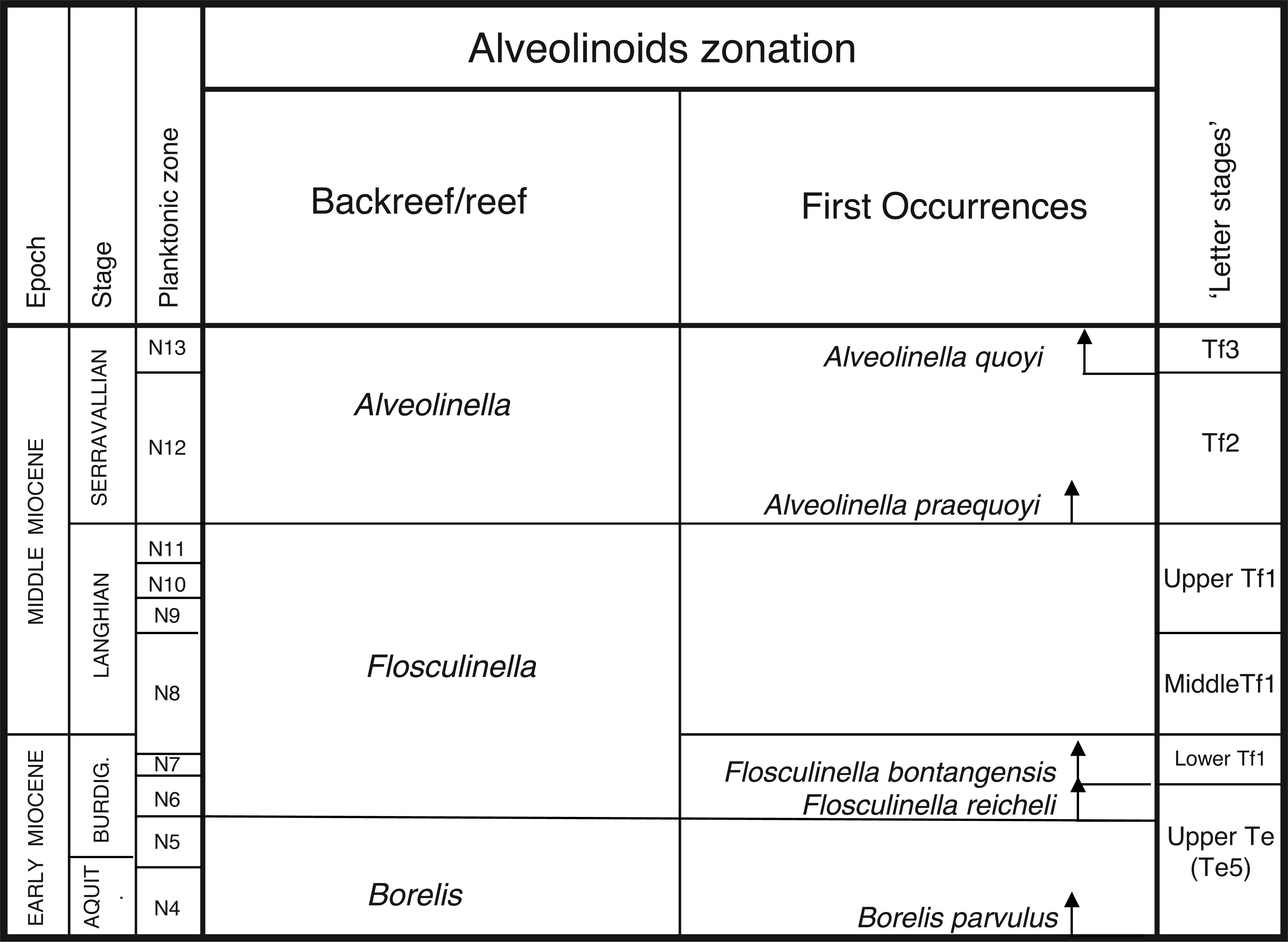
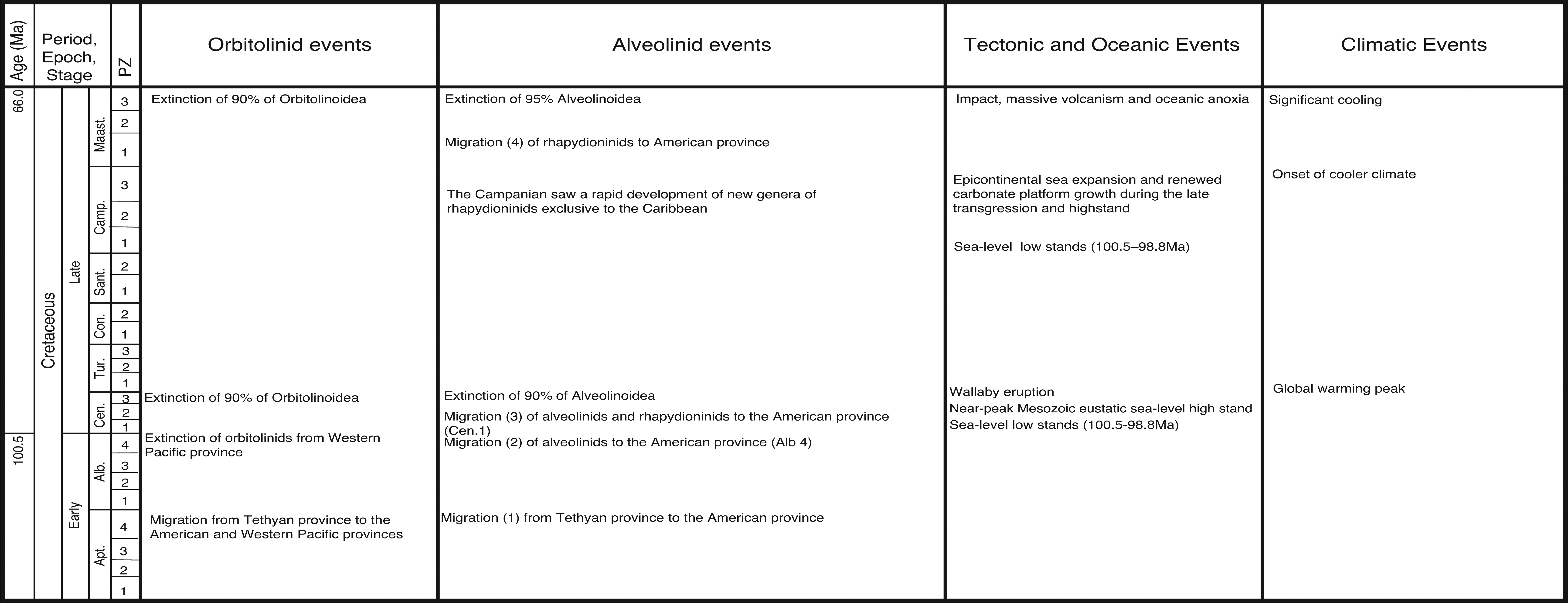
In this paper, we revise some of the analysis presented in BouDagher-Fadel [ 1], and present results from material recently obtained as a result of various investigations of LBF-bearing carbonate facies from two Atlantic basins, one from offshore Brazil (the Campos Basin), and the other off Venezuela (the Matacaibo Basin). These are augmented by studies of new material from several localities including from Saint Barthélemy (Lesser Antilles); Cardenas, San Luis Potosi, Mexico; the shallow marine sequences of the Ainsa Basin, Spain; Iles Madam and Audignon, France; Les Scaffarels sections from the Western Alps; the Circum-Troodos Massif sedimentary succession, northern Cyprus; the island of Paxos, Greece; Latakia, northwest Syria; Lebanon; the Kuh-I-Bingistan section and Savark formation, Iran; the Red Sea; the Pila Spi formations in Jabal Hiebat Sultan, Iraq; the Ladakh region, India; the Tingri and Gamba regions of southern Tibet; the Sekau formation, Indonesia; Port Moresby, New Guinea; the Sarawak Basin (onshore carbonates from Tinjar Province); and the Baden-Brickyard, Baden, Australia. The detailed locations of the materials studied can be found in associated cited references below. As a result, by combining our observations with those in the literature, we are able to propose a comprehensive, global, systematic analysis of the biostratigraphic, phylogenetic and palaeogeographic evolution of the alveolinoids. In our definitions of stratigraphic ranges, we primarily use the planktonic foraminiferal zonal (PZ) scheme of BouDagher-Fadel [ 24, 25], which is tied to the time scale of Gradstein et al. [ 26]. In this paper, the PZ scheme is also correlated with the LBF ‘letter stages’ of the Far East, as defined by BouDagher-Fadel and Banner [ 27], and later revised by BouDagher-Fadel [ 1] (see Figs 1 and 2), and with the biogeographic zonation of shallow benthic larger foraminifera as defined by Serra-Kiel et al. [ 16], which incorporates a series of biozones proposed for the Paleogene of Western Tethys based on species of Alvceolina, Nummulites and Assilina [ 28]. These biozones have been integrated into the shallow benthic zones (SBZ) covering the Paleocene to Eocene of the Mediterranean region by Serra-Kiel et al. [ 16], as a system of numbered units for the ‘Tertiary’, with SBZ 1–23 covering the Paleogene (see Fig. 1).
Alveolinoids Paleogene biozones with diagnostic first and last occurrences, calibrated against the PZ, the ‘letter stages’ of the Far East, and the ‘SB’ zones of the Mediterranean, modified after BouDagher-Fadel [ 1].
As described below, our analysis of the evolution and distribution of the alveolinoids will show that our previously developed hypothesis (i.e. that periodic, sea-level low stands enable inter-provincial migration of LBF [ 2, 7]) is again strongly supported, and that the behaviour and directions of migration of the alveolinoids is resonant with those of the orbitolinids [ 2], the orthophragminids [ 6], the nummulitoids [ 5], the miogypsinids [ 4] and the lepidocyclinids [ 3].
The alveolinoids originated in the Cretaceous in the Tethyan province. We conclude here for the first time that during a global sea-level low stand, migration of some alveolinoid species to the Americas occurred. As this province again became isolated as sea levels rose, parallel evolutionary trends between related forms of alveolinoids in the two re-isolated provinces subsequently occurred. After the end-Maastrichtian mass extinction, rare new forms of alveolinoids reappeared first in the Americas. Previous studies also suggested that the Americas were the region of the post-K–P appearance of the rotaliid LBF. As also previously inferred from our studies of a number of rotaliid LBFs, sea-level low stands in the Paleocene allowed some alveolinoid forms to migrate from the Americas to Tethys, where again later sea-level rises led to provincial isolation and parallel provincial evolutionary trends. As also occurred for the previously studied rotaliids, the alveolinoids flourished in Tethys and subsequently migrated from Tethys to the Indo-Pacific province, where they further diversified. With the closure of the Tethys seaway in the Miocene (late Burdigalian), the Mediterranean alveolinoids became isolated from their Indo-Pacific descendants and exhibited parallel evolutionary trends with more evolved, different lineages in the Indo-Pacific province showing morphological convergent trends similar to previous Cretaceous stock. Alveolinoids still exist today ( Borelis and Alveolinella), the former of which is cosmopolitan, while the latter is restricted to the Indo-Pacific province.
The Alveolinoidea throughout their phylogenetic history evolved morphologically divergent features, giving rise to identifiably distinct genera. As in previous biogeographical studies, however, we also confirm spatial and temporal gaps in their phylogenetic/stratigraphic distributions [ 29– 32]. We find that these gaps are best explained by homoplasy, in which convergent and characteristic features reappear as a result of either similar ecological evolutionary selection pressures, or a genetic predisposition to the repetition of specific mutations that produce morphological characteristics that are used to define a genus.
Morphological and paleoenvironmental characteristics of the Alveolinoidea
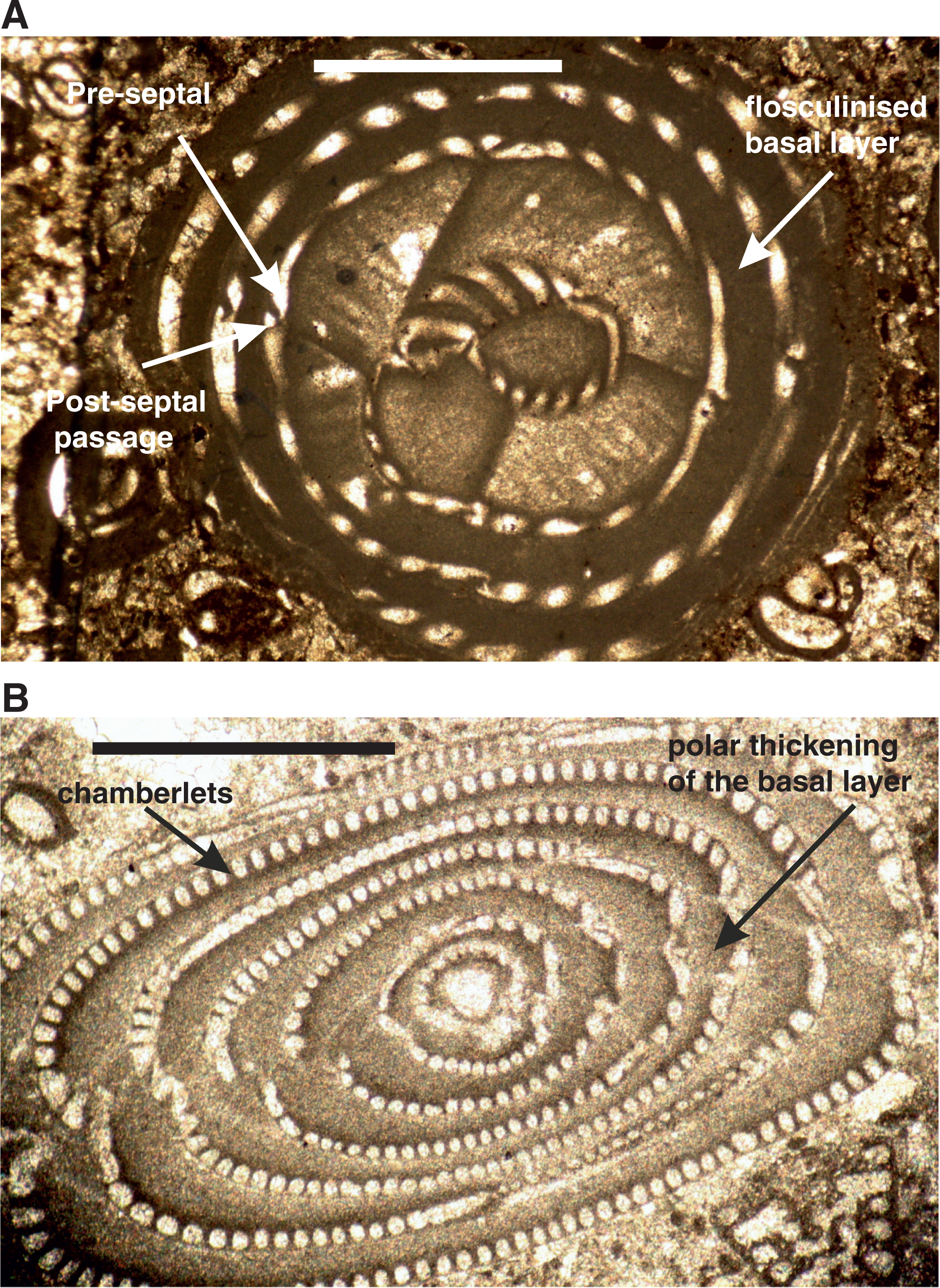
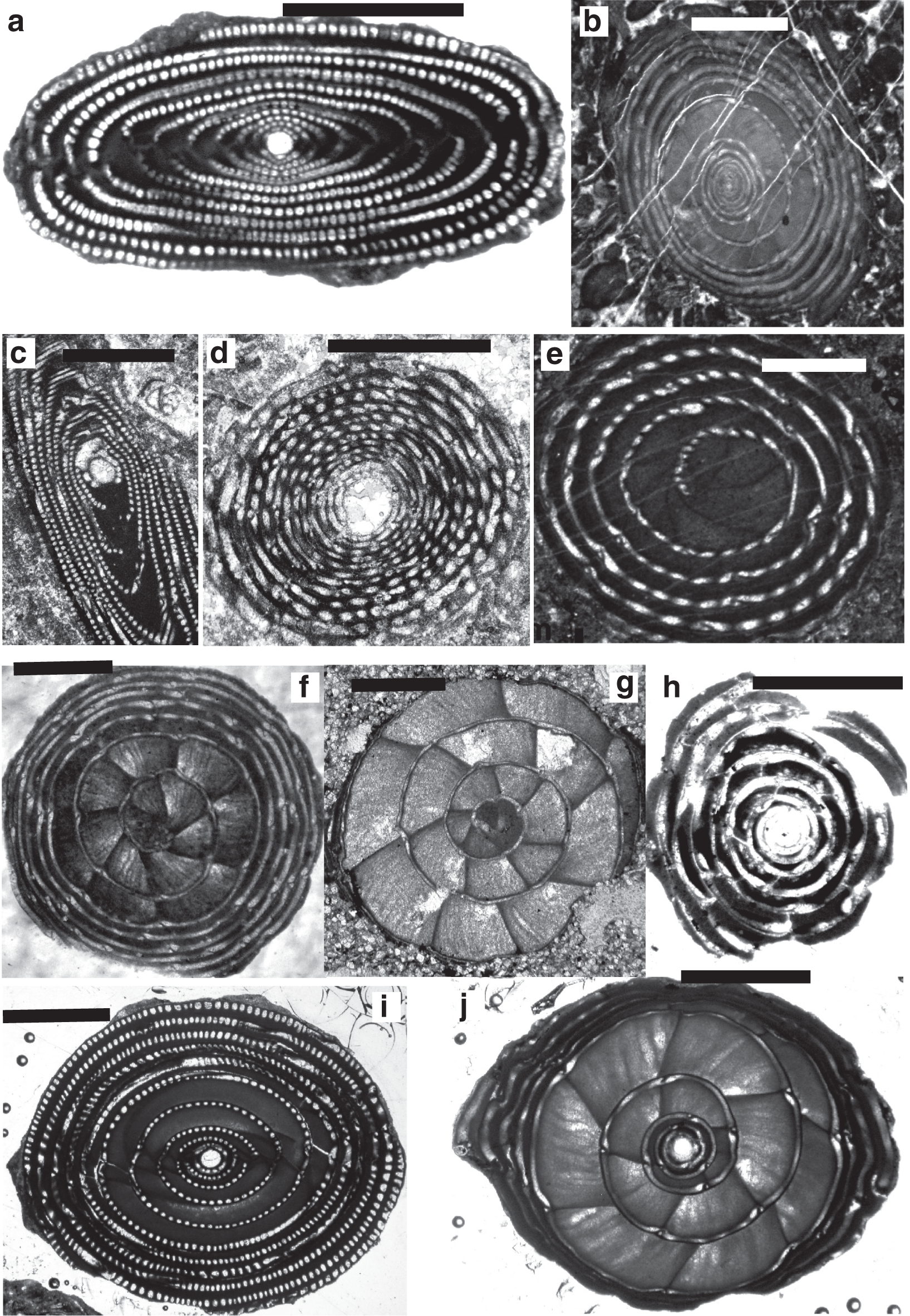
The Alveolinoidea made their first appearance in the Early Cretaceous ( Fig. 3) exhibiting a fusiform morphology. As is characteristic of miliolids, they have a porcelaneous, imperforate wall structure, with fine, randomly oriented crystals of high-magnesium calcite. Their tests are enrolled along an elongate axis, being initially planispiral or streptospiral, or ‘miliolid’, with chambers added in varying planes ( Fig. 4). In an extreme example of ecologically driven convergent evolution involving forms from different orders, the external appearance of alveolinoids closely resembles the planispiral, fusiform fusulinides of the late Paleozoic. This example of homoplasy is the result of ecological selection pressures acting on forms from different orders filling equivalent niches but in different stratigraphic periods. Many alveolinoids attained the same centrimetric scale as the fusulinides, but differ fundamentally in their imperforate, porcelaneous wall structure, and are indeed very distinct when studied in axial and equatorial (median) sections (see [ 1]). The basic morphology of the alveolinoids is shown in Figs 4– 7. Unlike the fusulinides, septal folding does not occur and the spiral septula reach the floor across the chamber. Deposition of secondary calcite (flosculinisation, Figs 4– 6) occurs more evenly across the floor of the chamber or may be restricted to a specific period of ontogenesis, rather than being almost entirely concentrated in the axial zone as in the fusulinides. The embryonic apparatus consists of a spherical proloculus followed by a spiral tube (flexostyle), succeeded in some genera by a miliolid nepion (pre-adult stage). In the adult, numerous chambers are planispirally coiled around an elongate axis, each chamber being broken into tubular chamberlets by secondary septa (septula) that run in the direction of coiling. Connectivity between the chambers is enabled through the vertical septula (canals/passages). These may be situated in front of the septum (post-septal) or behind it (pre-septal) (see [ 1]).
A) The evolution of the Alveolinidae from a simple streptospiral origin, such as Pseudonummuloculina; B) The evolution of the Rhapydioninidae from the lenticular compressed Pseudedomia; C) The evolution of the Fabulariidae from a completely overlapping form with vertical incomplete partitions, Periloculina.
In the Cenozoic, the alveolinoids re-appeared in the Paleocene seemingly independently from their Cretaceous relatives ( Fig. 8), but evolving from a common simple, long-ranging miliolid ancestor, in an example of homoplasy driven by a predisposition towards the occurrence of a repeated set of mutations that produce generic characteristics. The Paleocene forms share morphological characteristics with their Cretaceous relatives, and evolved separate parallel lineages in both the Tethyan province and the American province. As will be discussed in detail below, this repeated evolution from a simple miliolid common ancestor, such as Pseudonummuloculina caused homoplasic generic characteristics, such as alveoles, to reappear on numerous occasions in the Paleogene and in the Neogene.
In the Middle Eocene, alveolinoids reached morphologically large sizes (e.g. Alveolina prorrecta Hottinger) growing up to 3.5 cm in the diameter of the axial section [ 8]. Their sizes were made achievable as a result of the evolutionary development of a variety of morphological structures that had the effect of mechanically strengthening the alveolinoid test, such as by the thickened basal layer (which is subdivided by pillars or secondary partitions in the Fabulariidae), the canaliculation and thickening of the central zone except for a pre-septal space with buttresses or residual pillars (as in the Rhapydioninidae), and the flosculinisation with thick deposition of secondary calcite (as in the Alveolinidae [ 1]). The basal layer of the larger foraminiferal test, when thickened by flosculinisation [ 8] or perforated by canalicular passages [ 33], exists only in the porcelaneous forms ( Figs 5– 7).
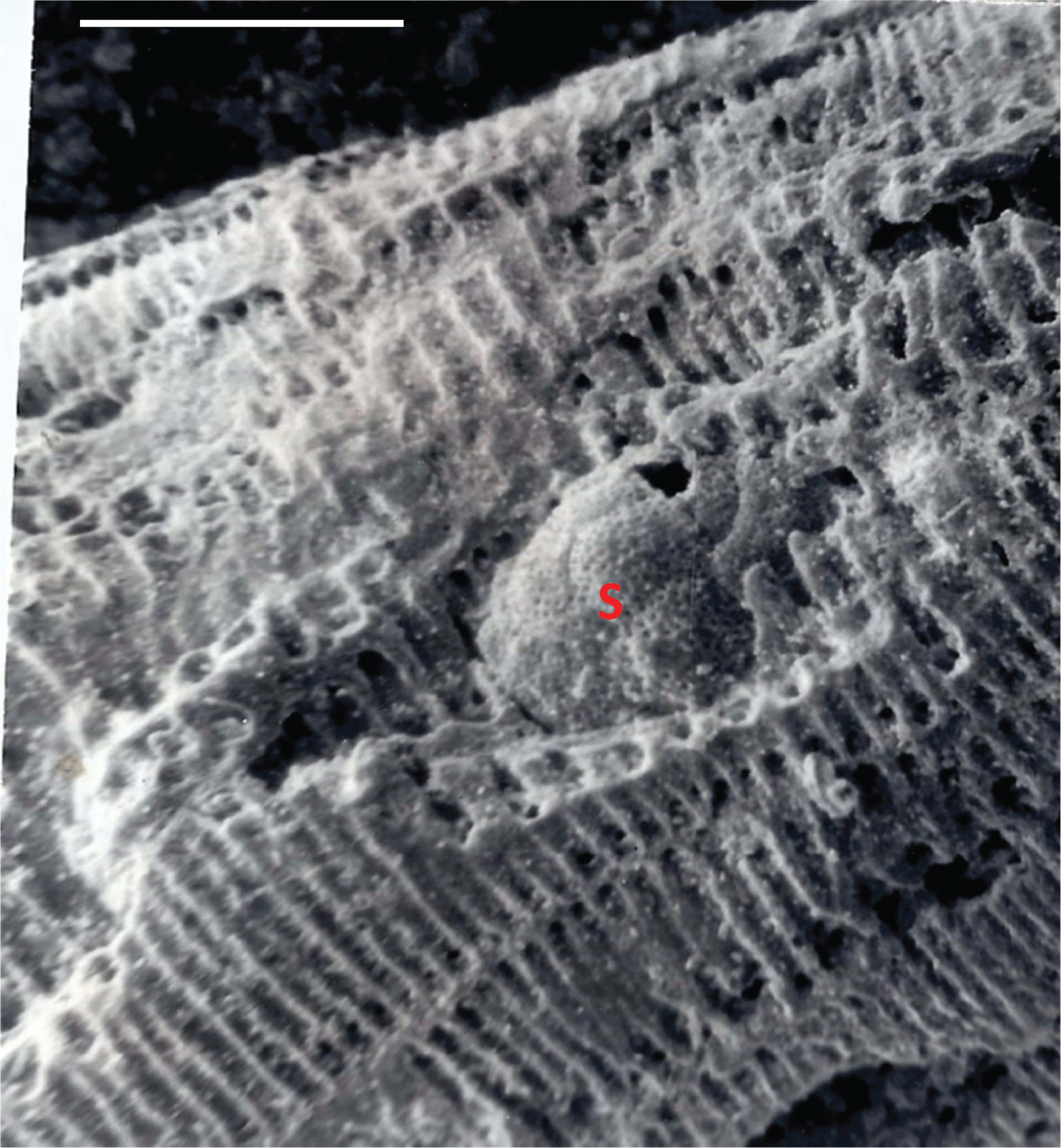
The development of alveoles (blind recesses separated by septula; e.g. see Fig. 6) in some alveolinoids may have allowed them to harbour photosymbionts (e.g. diatom symbionts in living Alveolinella Douvillé [ 34], see Fig. 9). Endosymbiosis apparently played a major role in the evolution and diversification of the alveolinoids, and appears to be a key innovation that facilitated a change in habitat from an epifaunal, free-living mode of life, to one of living attached to phytal and non-phytal substrata.
Alveolinella quoyi (d’Orbigny), Port Moresby, Coral Sea, New Guinea, solid specimen figured by BouDagher-Fadel [ 1], showing a parasite-symbiont(s) completely enclosed by the whorls, Planorbulinopsis parasitica Banner, embedded in the test. Scale bar = 0.25 mm.
On the other hand, the development of pillars in the fabulariids (see Fig. 7) and rhapydioninids may also be seen as providing mechanical strength to the test. For example, in discoidal forms, heavily pillared endoskeletons, as a rule, occur in forms living in very shallow, turbulent water. In the present day, robust and fusiform tests (as seen in Alveolinella) are adapted to a life in environments of moderate hydrodynamic energy, characteristic of water depths of less than 30 m, usually not in mobile sands, but rather on phytal or hard substrates such as reef rubble [ 1, 35]. At Lizard Island, Australia, sand patches at ~15–20 m seemed to be the best habitat to collect Alveolinella (Hallock, personal comment). According to Hottinger [ 36], in Alveolina the function of the elongated fusiform test is related to motility, the test growing in the equatorial direction but moving in the polar direction. The living alveolinids (such as the elongate Alveolinella) appear to be confined to shallow-marine (down to depths of 80 m), clear, well-oxygenated, tropical and subtropical waters. They can hide or shelter under shallow layers of coral sand, in order to regulate the illumination that they require. Therefore, in comparing the occurrences of the extinct alveolinids with their Holocene descendants, we can safely deduce that these fusiform types, with large alveoles, thrived in sediments deposited in warm, shallow water. In fact, all alveolinoids are regarded as neritic (inner shelf) by Reichel [ 37], restricted to littoral, tropical, protected shelf and reef shoals [ 1, 30, 38], or moderate energy zones of the shallow ramp [ 39], and some genera are epiphytic [ 40].
Phylogeny and palaeogeographic distribution of the Alveolinoidea
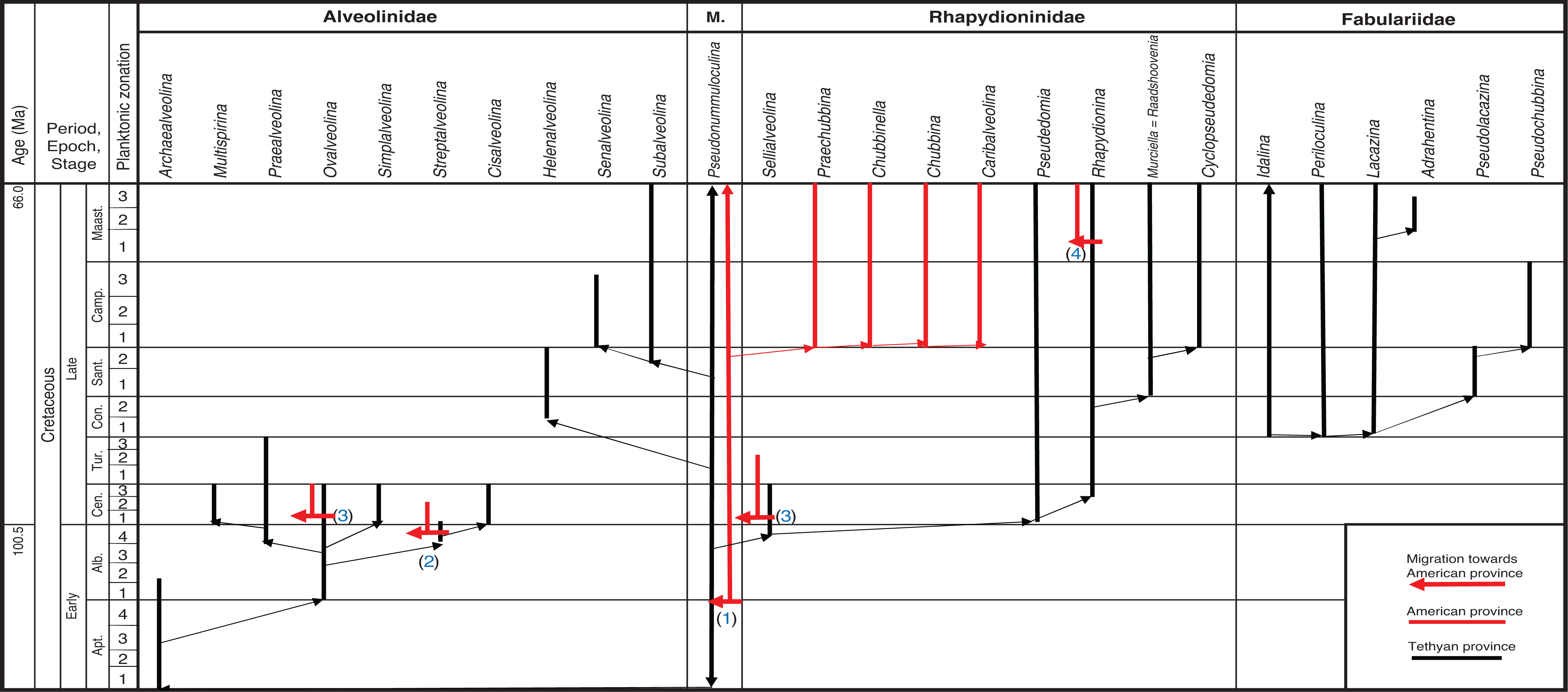
In this section, we will review the phylogenetic development and taxonomic relationships of the alveolinoids of the Cretaceous, Paleogene and Neogene. Furthermore, we clarify their provincial distributions, based around the Tethyan, American and Indo-Pacific provinces. We discuss the development of the three alveolinoid families, the Alveolinidae, the Fabulariidae and the Rhapydioninidae, which in the Cretaceous occurred in the Tethyan and American provinces, and then the later Cenozoic forms, of which the Alveolinidae and the Fabulariidae occurred in all three provinces (Tethys, American and the Indo-Pacific), while the Rhapydioninidae were constrained to the Americas.
The Cretaceous Alveolinoidea
The Tethyan Province
The limestones of the Tethyan province range from what is today the Western Mediterranean, through the Middle East to the carbonate platforms of Tibet. This province saw the first development of the alveolinoids from small simple miliolids, such as Pseudonummuloculina (see Fig. 3). This latter is a simple small streptospiral-involute porcelaneous form, with an apertural slit or with a row of multiple apertures. It made its first appearance in the latest Hauterivian (PZ Hauterivian 2 [ 1, 41]). It was widely distributed in the Tethyan province from the Albian until the early Paleogene [ 32, 42– 47], and is the ancestral form for many of the Alveolinoidea.
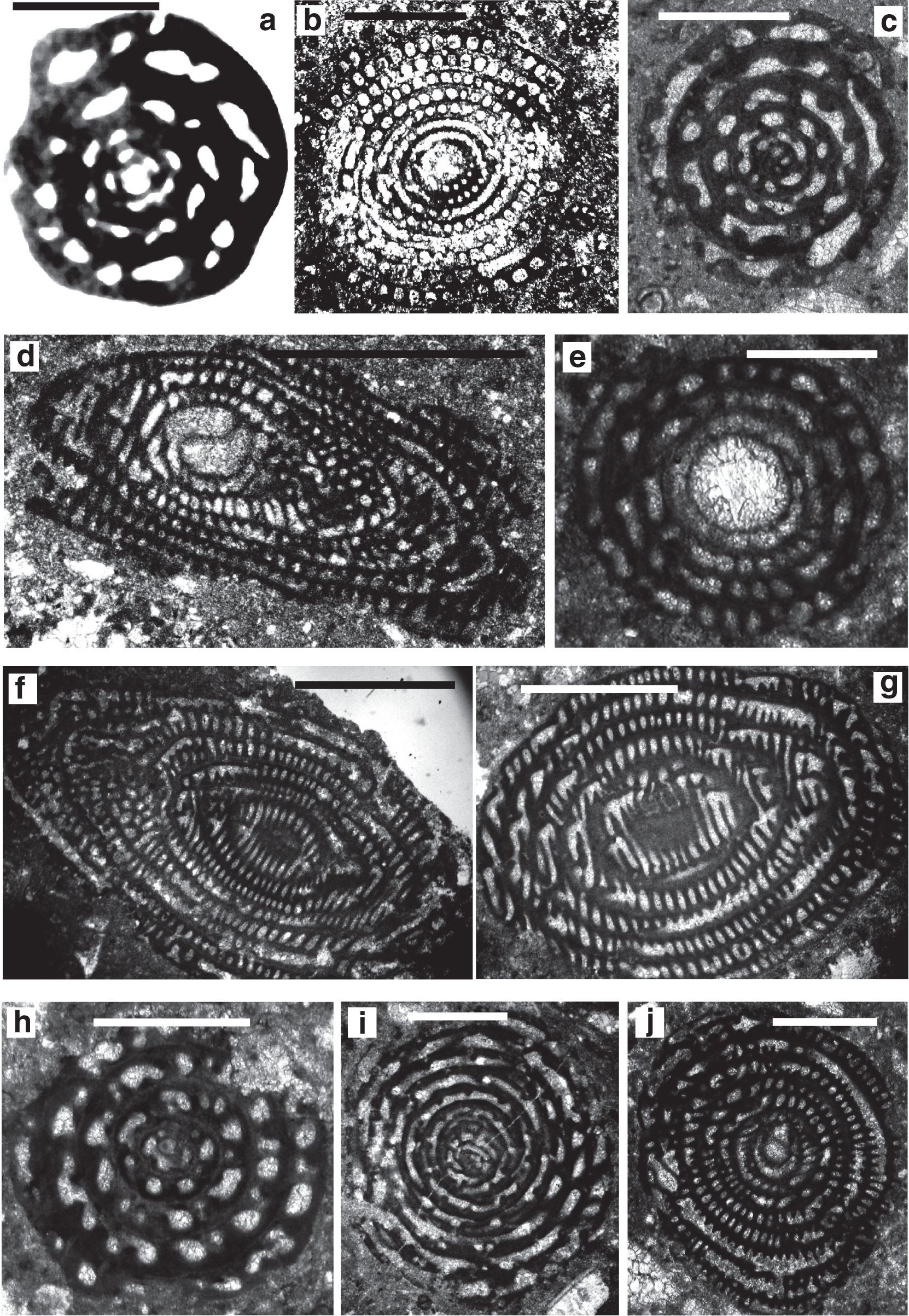
Archaealveolina ( Plate 1, a) is the earliest defined Alveolinidae. It is subglobular with an early streptospiral stage, pre-septal passages and a single row of apertures at the base of the apertural face. This genus first appeared at the beginning of the Aptian and disappeared at the end of PZ Albian 1 [ 44]. Archaealveolina gave rise to and was replaced in the Albian by Ovalveolina ( Plate 1, b–c), a globular form with planispiral coiling and large pre-septal passages [ 1]. Ovalveolina is recorded from many Tethyan localities ranging from PZ Albian 1 to PZ Cenomanian 3, but as discussed below, is not found in the American province until the beginning of PZ Cenomanian 2.
Scale bars: a, c, f, h = 0.5 mm; b, d, e, g, i, j = 1 mm.
a. Archaealveolina reicheli (de Castro), figured by Loeblich and Tappan [ 48], Aptian, Italy.
b. Ovalveolina sp., Cenomanian, France, UCL collection.
c. Ovalveolina crassa Castro, Cenomanian, Iles Madam, UCL collection.
d. Praealveolina cretacea Reichel, Cenomanian, Iles Madam, UCL collection.
e–f. Praealveolina tenuis Reichel, Cenomanian, e) oblique equatorial section; f) oblique axial section, Audignon, near Saint-Sever, UCL collection.
g–j. Simplalveolina simplex (Reichel), Cenomanian, Audignon, near Saint-Sever, UCL collection.
In PZ Albian 4, the planispiral Ovalveolina gave rise to subglobular/fusiform Praealveolina ( Plate 1, e–f) with much lower and narrower chamberlets. The apertural face of Praealveolina has, as does Ovalveolina, a single row of pores near the equator, however, the pores increase poleward to many rows of openings. Simplalveolina ( Plate 1, g–j) which also appeared at PZ Cenomanian 1, is small and ovoid with numerous aligned septula, but lacks secondary chamberlets. The aperture is a single row of pores in the apertural face. In PZ Cenomanian 1, Praealveolina gave rise to the spherical Multispirina ( Plate 2, a), with large, numerous chambers, interleaved by spires [ 37]. The aperture is formed by a row of pores in the apertural face of each of the multiple spires. The growth of Multispirina is an example of an alternative to elongate growth, where the multiple spires are a means of accelerating its growth [ 49]. The multiple spires also enhance the internal inter-connectedness of the pore spaces, as the numerous small apertures along the sutures connect the whorls in an irregular manner, thus making for short radial lines of interconnectedness throughout the test [ 33].
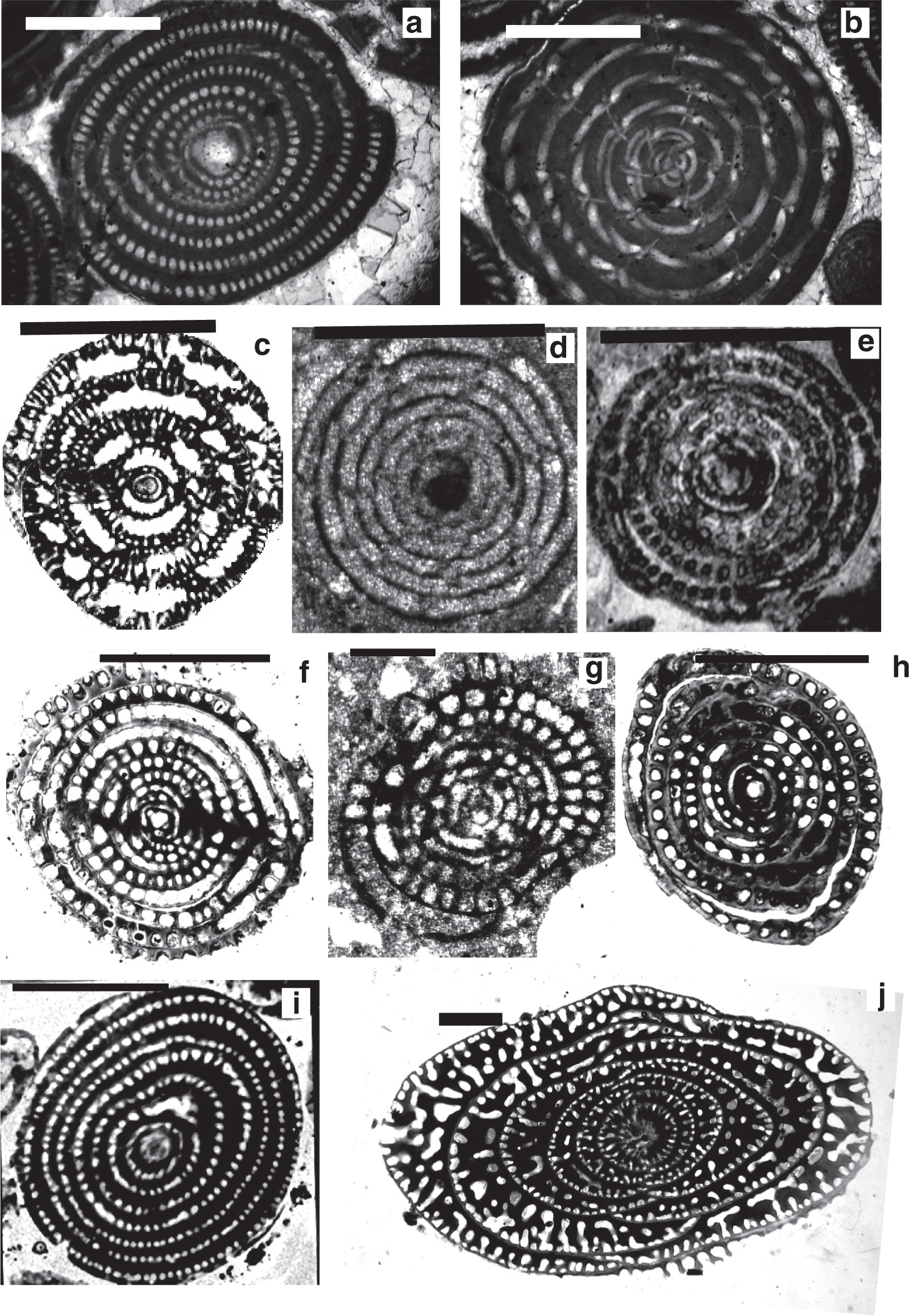
Scale bars: a = 0.15 mm; b–g, i, k = 0.5 mm; h, j = 1 mm.
a. Multispirina sp., Cenomanian, Qatar, NHM M2425.
b. Streptalveolina sp., Cenomanian, Iles Madam, UCL collection.
c–f. Cisalveolina lehneri Reichel, Cenomanian, India, UCL coll.
g. Pseudonummuloculina sp. Cenomanian, Paxos, Greece, P. Scholle coll.
h. Pseudedomia persica Rahaghi, Cenomanian, Iran, Nanjing University coll.
i. Helenalveolina tappanae Hottinger, Drobne and Caus, Cenomanian, Iles Madam, UCL collection.
j–k. Pseudochubbina globularis (Smout) (= Pseudedomia globularis Smout), paratypes, Campanian, Iraq, NHM P42646.
Parallel to the Praealveolina lineage, Ovalveolina also gave rise to the small to medium size globular, streptospirally coiled Streptalveolina ( Plate 2, b; PZ Albian 4–Cenomanian 2). The latter also has large pre-septal passages and a single row of apertures on the apertural face. The trend in this lineage is to evolve planispiral later whorls, as in Cisalveolina ( Plate 2, c–f; PZ Cenomanian 1–3), which has alternating septula with a simple narrow, slit-like aperture extending from pole to pole. Cisalveolina, unlike all the Cretaceous genera with pre-septal canals, has post-septal passages.
Of the early alveolinids, 85% did not survive the widespread oceanic anoxic event (OAE) at the Cenomanian–Turonian boundary (93.9 Ma), which may in turn have been triggered by the Madagascar and Caribbean volcanic provinces events ( Fig. 10). The only survivor was Praealveolina, which in turn went extinct at the end of the Turonian.
The alveolinids re-appeared in the Late Cretaceous of the Tethyan province, but notably those that did appear had a streptospiral early stage and a planispiral-involute adult stage. It has been proposed that they are again phylogenetically linked to Pseudonummuloculina ( Plate 2, g), and are an example of the homoplasic, re-appearance of morphologically identical form from a long ranging, robust small-form common ancestor [ 1, 15]. They include the spherical Helenalveolina ( Plate 2, i; PZ Coniacian 2–Santonian 2), Subalveolina (PZ Santonian 2–Maastrichian 3), and the axially elongated Senalveolina in the early Campanian. Helenalveolina is distinguished from the Fabulariidae by the absence of pillars subdividing the chambers, by its streptospiral coiling, and by the lack of a trematophore in its apertural face.
The earliest Rhapydioninidae originated in Tethys at the end of the Albian. The axially compressed rhapydioninids have homologous features to the alveolinids, and occupied similar niches, but developed separate parallel lineages. They evolved, as did the alveolinids, from the streptospiral Pseudonummuloculina, but did so later than the first appearance of the alveolinids [ 1, 15, 46, 50]. They had a planispiral to streptospiral early stage, but diverged from the alveolinids lineages by becoming uncoiled, compressed, peneropliform-flaring, or cylindrical in later stages. The basal layer of the rhapydioninids is pierced by tubular supplementary passages as part of the ‘central thickening’ [ 51]. The rhapydioninid are comparable to modern peneroplid foraminifera, which commonly are found in shallow-water epifaunal habitats or on epiphytal hard substrates, including seagrasses and algal thalli [ 52– 55].
Sellialveolina ( Plate 3, a–b), was the most primitive rhapydioninid and occurred across Tethys between PZ Albian 4–Cenomanian 4 [ 17, 56]), colonising the carbonate platforms from the Middle East to the Iberian Peninsula. Unusually, it is a cosmopolitan rhapydioninid, and is reported from the American province from PZ Cenomanian 2–Turonian 2 (n.b. in the Maastrichtian, Rhapydionina becomes the only other example of a cosmopolitan rhapydioninid). Sellialveolina has a lenticular, planispirally-enrolled test, where the chamber lumen is subdivided into numerous chamberlets by septula. Sellialveolina species increased in overall test size and complexity of internal structures during their stratigraphic evolution.
Scale bars: a–i, k–l = 0.5 mm; j = 1 mm.
a–b. Sellialveolina viallii Colalongo, Cenomanian, Xinyu, Nanjing University coll.
c. Murciella cuvillieri Fourcade, figured by Fourcade [ 63], Campanian, Spain.
d–e. Periloculina iranica Rahaghi, Cenomanian, Iran, Nanjing University coll.
f. Lacazina sp., Coniacian, India, UCL coll.
g–h. Chubbina cardenasensis (Barker and Grimsdale), g) Late Cretaceous, Cardenas, San Luis Potosi, Mexico, NHM P33044; h) figured by Robinson [ 10] as a co-type megalospheric specimen of Chubbina jamaicensis Robinson, Campanian, Jamaica, NHM P48053.
i. Glomalveolina pilula Hottinger, Ypresian, Lower Laki Formation, Pakistan, UCL coll.
j. Glomalveolina sp., Eocene, Libya, UCL coll.
k. Glomalveolina lepidula (Schwager), Early Eocene, Longjiang section, Tibet, Nanjing University coll.
l. Pseudofabularia matleyi (Vaughan), figured by Loeblich and Tappan [ 48], Middle Eocene, Chapelton Formation, Jamaica.
Tethyan rhapydioninids are provincially characterised by Pseudedomia ( Plate 2, h) and its descendants, which evolved from Sellialveolina in PZ Cenomanian 1. They are common in the Late Cretaceous deposits of the European–North African region [ 51, 57– 62]. This lineage exhibits an evolutionary trend with an increasing diameter of the megalospheric form, a reduction in the streptospiral nepionic stage, and an increase in the differentiation and regularity of the peripheral chamberlets in comparison with the median chamberlets in the same chamber [ 50].
Sellialveolina and Pseudedomia both resemble Ovalveolina in their globular initial part, but develop compressed, curved chambers in their final whorls. The chambers in Pseudedomia become progressively longer, strongly overlapping the preceding chambers. The evolutionary lineage from Pseudedomia showed progressive uncoiling and cylindrical development through Rhapydionina, Murciella ( Plate 3, c) and Cyclopseudedomia (see Fig. 7). The ‘central thickening’ of Rhapydionina compares with the thickened basal layer in the polar area of Subalveolina [ 64]. Murciella has crosswise, oblique, median tubular chamberlets (the ‘helicoidal’ structures of Fleury [ 65]), while Cyclopseudedomia is cyclical with compressed arched chambers in its final whorls.
We believe that the Tethyan Murciella has sometimes been misidentified as a Raadshoovenia, which in fact is an American Paleocene form (see below). For example, the Late Cretaceous Tethyan Raadshoovenia [e.g. R. salentina (Papetti and Tedeschi)] has the same advanced early planispiral-involute nepiont as Murciella, and the two genera were considered synonyms by De Castro [ 57]. We agree with this analysis and contend that all Cretaceous, Tethyan Raadshoovenia are in fact synonyms of Murciella.
All Tethyan rhapydioninids, including Murciella, went extinct at the end of the Maastrichtian. The true Raadshoovenia appeared in the Paleogene of the American province. There are no records of Raadshoovenia from the Paleocene of the Tethyan province. Raadshoovenia has a streptospiral nepiont in both megalospheric and microspheric generations, and characteristically has a smaller test than Murciella. Murciella and Raadshoovenia evolved in different times on the two sides of the Atlantic. They are again an example of homoplasic evolution, rather than representing a single genus that survived the events at the Cretaceous–Paleogene boundary while simultaneously moving provinces.
The Fabulariidae are also common in similar ecological niches as the alveolinids, but they did not appear before the Late Cretaceous and were endemic to the Tethyan province, with no record from the Cretaceous deposits of the American province. They arose directly and independently from the alveolinids and the rhapydioninids, either from the simple Quinqueloculina group [ 66], or they had a true miliolid origin from, for example, the simple-form Idalina (see [ 1]). The fabulariid test is large, dimorphic, multi-chambered with miliolid coiling, tending to become reduced in subsequent growth stages, either to bilocular or to monolocular chamber cycles, with a trematophore as the aperture. According to Hottinger ([ 67], p. 4) ‘A trematophore, or a sieve constituting the face of many porcelaneous larger foraminifera, is in miliolids produced by the coalescence of teeth, covering a large pre-septal space. It may be supported by residual pillars’. Chambers have a thickened basal layer, subdivided by pillars or secondary partitions. Foraminifera in the adult growth stages have a fixed apertural axis. For the terminology and the orientation of sections needed for detailed structural analysis see Drobne [ 68] and Hottinger et al. [ 15].
Idalina, a simple miliolid with a quinqueloculine to biloculine test, gave rise to Periloculina ( Plate 3, d–e; see [ 15]), which has incomplete partitions and an aperture consisting of a round trematophore with a bar-like tooth. Also, in the Coniacian, Lacazina ( Plate 3, f) appeared, with chambers that overlap throughout, with a concentric growth pattern, and with an annular trematophore ( Figs 3 and 7) [ 1, 69]. Lacazina is reported from many places in the Tethyan province, for example, from Southern Europe [ 32, 38], France [ 48, 60, 70], Spain [ 70– 72], Sardinia [ 15, 60], and Israel [ 48, 71, 72]. In Pseudolacazina the chambers are subdivided by longitudinal partitions or by pillars supporting the chamber roof, while in Pseudochubbina ( Plate 2, j–k) parallel anastomising passages are present in the basal layer. Correcting BouDagher-Fadel [ 1], we now believe that all Cretaceous fabulariids, except the small, primitive, robust Idalina, went extinct at the K–P boundary.
The American Province
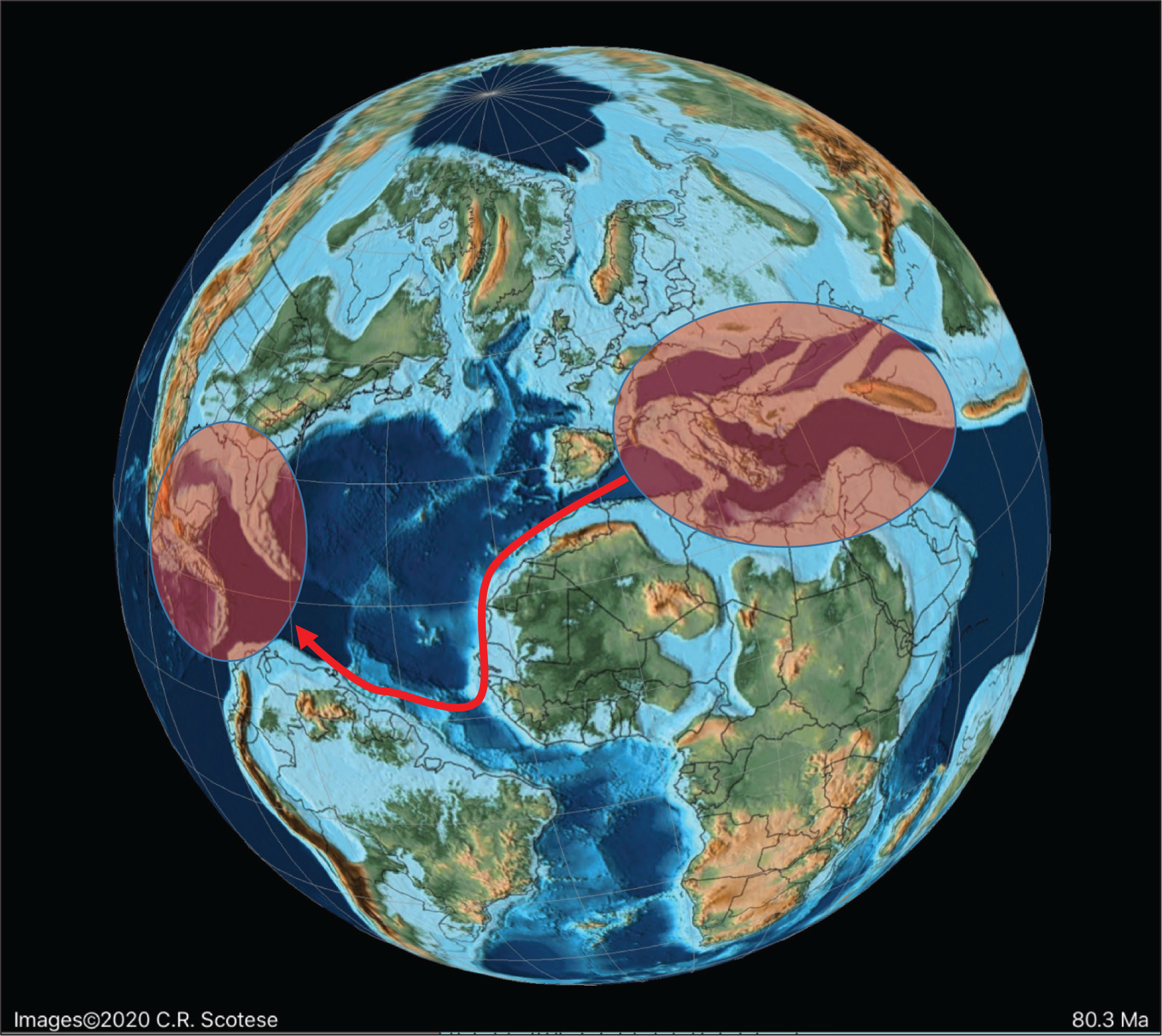
The mid-Cretaceous Alveolinidae were extremely rare in the American province, and post-date the Tethyan forms. It is inferred that they migrated from the Tethyan province after their first appearance there. The pre-existing Tethyan Pseudonummuloculina appeared in the Americas together with some Tethyan orbitolinds (see [ 2]; Fig. 3) during the sea-level low stand of the Aptian–Albian boundary (migration 1 in Fig. 11), and is represented in both provinces by P. heimi (Bonet). This species is widely distributed in the Albian–Cenomanian of both Western Tethys and the Gulf Coast [ 74– 76]. It has also been recorded from Texas, Florida and Louisiana in the United States [ 77].
Variation in sea level during the mid to Late Cretaceous based on Miller et al. [ 73]. The red diamonds represent the planktonic foraminiferal zones, while the numbers signify the main migration events of the alveolinids.
The streptospiral alveolinids appeared in the American province, in PZ Albian 4b, and are represented by Streptalveolina mexicana Fourcade, Tardy and Vila [ 78]. This species initially described from the early Cenomanian, Mexican Aurora Formation, has also been recorded in some localities from PZ Albian 4 to Cenomanian 2 of the Gulf Coast [ 79, 80]. It is endemic to the American province, but appears to be virtually identical to the PZ Albian 4a, Mediterranean species S. peybernesi [ 81], and seems to have appeared in America after the late Albian sea-level low stand (migration 2 in Fig. 11). Finally, the pre-existing Tethyan Ovalveolina maccagnoae De Castro is reported from the Americas from PZ Cenomanian 2 [ 82] from the mid-late Cenomanian deposits of the Manantlan Range in Colima State, Mexico, which again correlates with a global sea-level low stand (migration 3 in Fig. 11).
In the American province, the Rhapydioninidae are of two types, represented by the mid-Cretaceous Sellialveolina, and the distinct, later Cretaceous Chubbina, Chubbinella, Praechubbina, Caribalveolina lineage.
Sellialveolina is the most primitive genus of the axially compressed Alveolinoidea, and is the only genus of the Rhapydioninidae that is found in the mid-Cretaceous of the American province. It first appeared as a Tethyan form in PZ Albian 4, but it is reported in the Americas 3.4 Ma later (in PZ Cenomanian 2) as Sellialveolina sp. and Sellialveolina cf. drorimensis (a Tethyan form) from northern Peru [ 83]. This appearance in the Americans coincides with that of Ovalveolina, which as noted above correlates with a global PZ Cenomanian 2, sea-level low stand (migration 3 in Fig. 11).
The later American rhapydioninids forms have no Tethyan equivalents, and seem to have evolved directly from the American Pseudonummuloculina. They are described from several localities in southern Mexico, Guatemala and in the Caribbean [ 10, 18, 84– 86].
They appear in PZ Campanian 1, and rapidly develop the Chubbina ( Plate 3, g–h) lineage, which is endemic to the American province. The most primitive genus of this lineage, Praechubbina, with a poorly developed endoskeleton, formed by short, incomplete septula and floors, is characterised by a single aperture in its early stages. It differs from the ancestor genus Pseudonummuloculina by the presence of endoskeletal elements and multiple apertures in adult stages of growth [ 18, 84]. The septula in Chubbinella and Chubbina are complete, and divide the chambers into long tubular chamberlets. The septula of Chubbinella form chamberlets of equal calibre within the same chamber, while in Chubbina the chamberlets are unequal and the median chamberlets are parallel to the peripheral chamberlets. In Chubbina the subdivision of the chambers begins in the early stages of growth, whereas in Chubbinella the early parts are undivided [ 17]. On the other hand, the axially elongated Caribalveolina has alveoles and chubbinid features in the poles that are similar but less regular than the polar features of Praealveolina [ 50].
A Late Cretaceous migration of the rhapydioninids from the American province to Tethys never occurred. However, towards the middle Maastrichtian (PZ Maastrichtian 2), migration of the Tethyan form Rhapydionina to the American province did occur, with Rhapydionina cf. R. liburnica (Stache) reported from PZ Maastrichtian 4 of south east Mexico [ 86]. The early Maastrichtian low sea-level stands (migration 4 in Fig. 11) correlate with the timing of this apparent trans-oceanic migration ( Fig. 12).
All American rhapydioninids went extinct at the K–P boundary.
The Paleogene Alveolinoidea
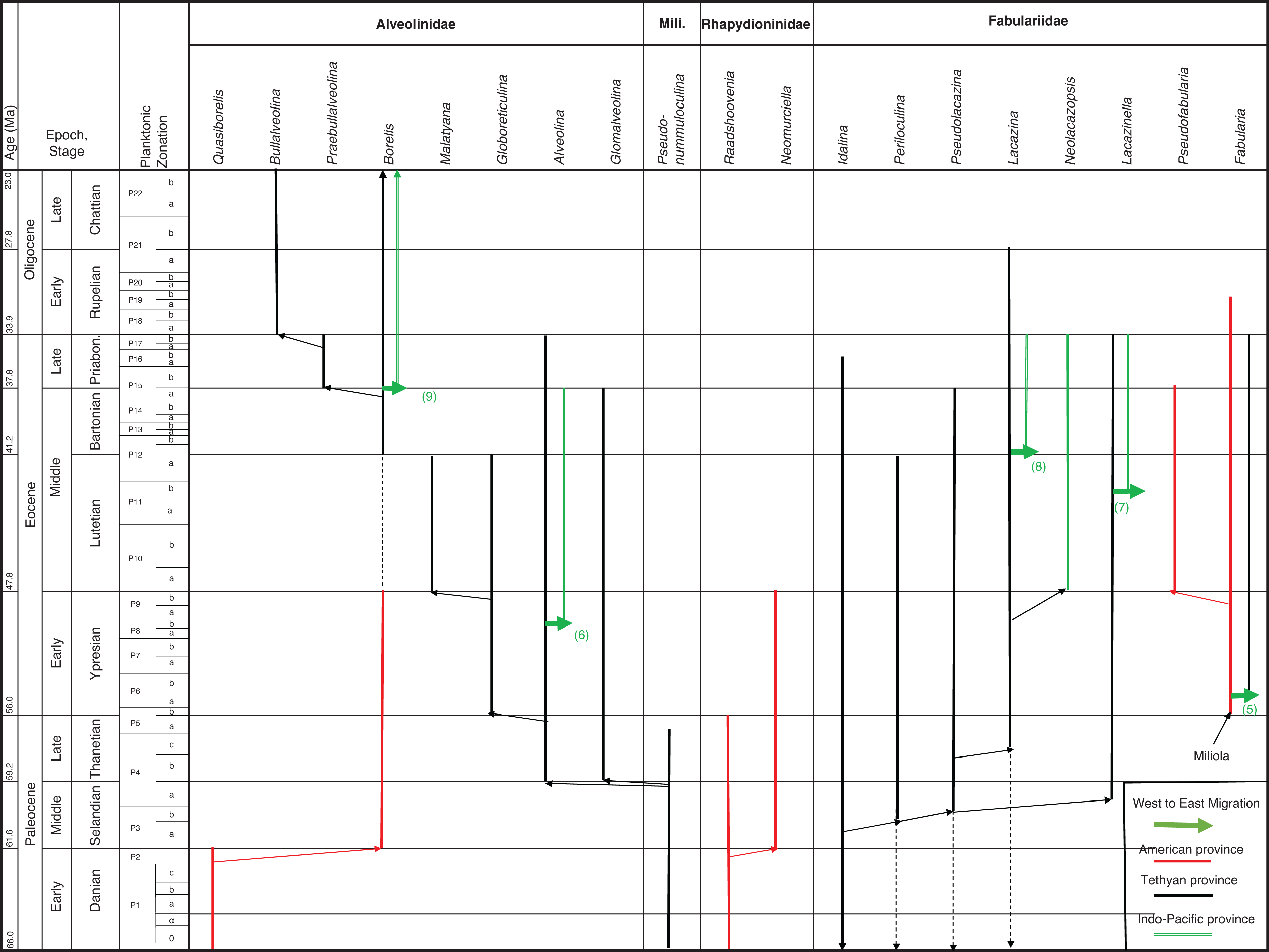
No complex Alveolinoidea survived the Cretaceous/Palaeogene (K–P) extinction, however their ancestral small and simple miliolids were robust enough to continue ( Fig. 8). In the Paleogene, these ancestral forms again gave rise to new members of all three of the Alveolinoidea families, which closely echoed their Cretaceous predecessors. The alveolinids of the Paleogene show a complex history of regeneration from the simple Pseudonummuloculina (which survived the end Cretaceous event and only went extinct in the late Thanetian, P4), with new forms occurring independently in both the Americas and Tethys. In the Eocene, migration of alveolinids again occurred, but as previously seen in our studies of the rotaliids, now in a west to east direction, with migration of forms from Tethys to the Indo-Pacific.
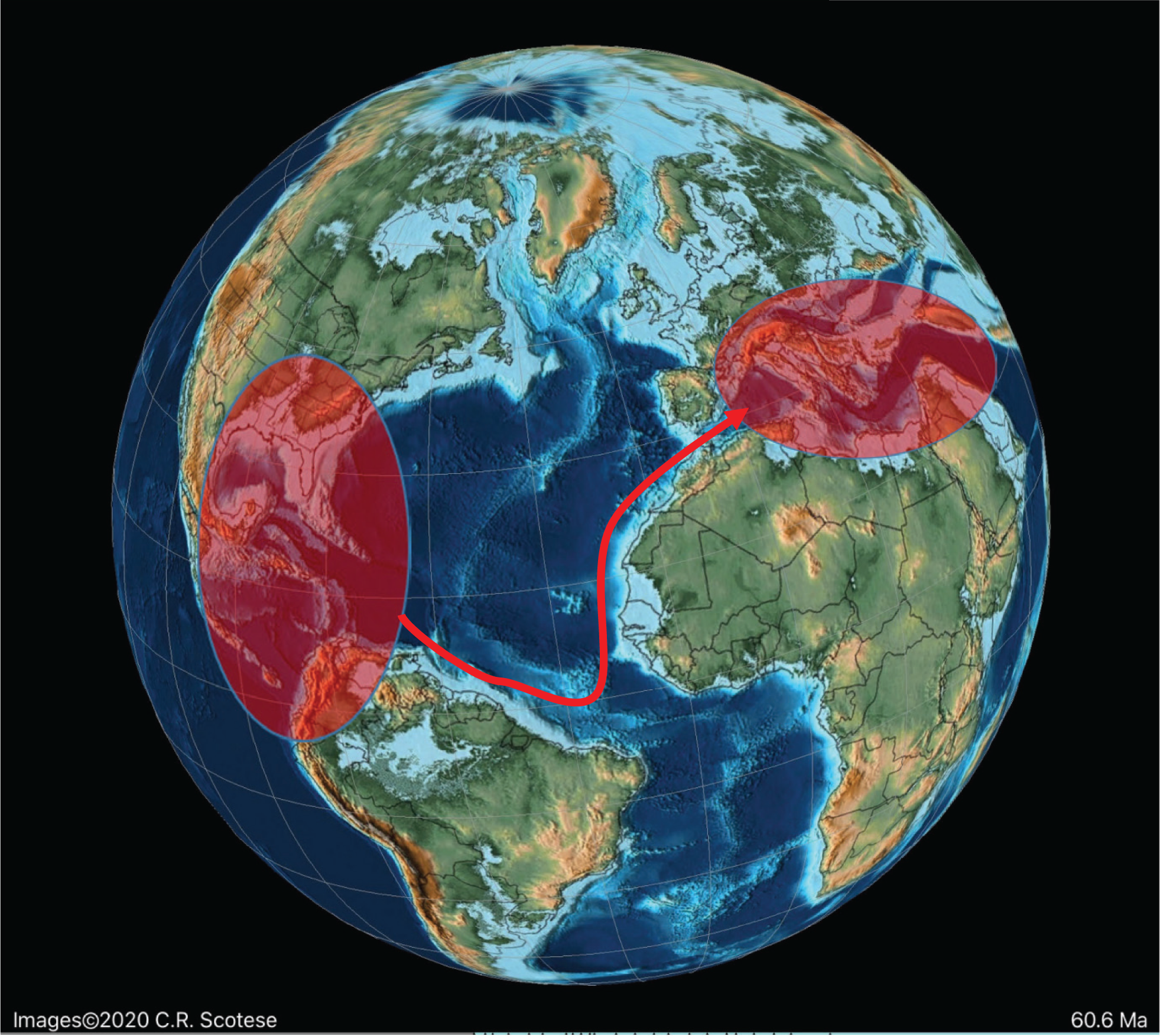
In the Paleocene, the rhapydioninids also re-appeared from a small miliolid after the K–P extinction, but are only found in the Americas ( Fig. 8). In Tethys the fabulariid ancestral form Idalina survived the Maastrichtian extinction, and gave rise to a second wave of Paleocene forms. They gave rise in the Middle Paleocene to a Periloculina lineage, which echoes the homomorphic Late Cretaceous lineage, but which is separated by a stratigraphic interval of over 6 Ma. In yet another example of homoplasy, Fabularia evolved in the Americas independently of the other Tethyan fabulariids, and migrated during an early Ypresian sea-level low stand to Tethys ( Figs 13– 15). All rhapydioninids and fabulariids went extinct before the end of the Paleogene, and only an alveolinid, Borelis, survived into the Neogene ( Fig. 16).
Variation in sea level during the Paleogene based on Miller et al. [ 73]. The red diamonds represent the planktonic foraminiferal zones, while the numbers signify the main migration events of the alveolinids.
The American Province
The alveolinids, except for a couple of primitive genera (not discussed in [ 1]), are rare in the Paleogene of the American province. A primitive small globular planispirally, coiled form, Quasiborelis Hanzawa [e.g. Q. gunteri (Cole)] with streptospiral early chambers, was reported by Hanzawa [ 87] from Florida ( Fig. 8). It has a simple internal structure, consisting of a poorly developed endoskeleton divided by few septula and floors, and high pre-septal canals, and it must have evolved from a small miliolid that survived the K–P event. Quasiborelis was short ranged, but was followed directly by forms similar to the later Tethyan Borelis (see [ 19]). The American Borelis is subglobular in shape with only one row of chamberlets and a planispiral later stage, and has been reported from the Middle Paleocene to the Early Eocene (PZ P3–P9) of the American province [ 18, 19, 88– 90]. The American Borelis occupies similar niches as the Tethyan Glomalveolina ( Plate 3, i–j; [ 19]), which is abundant in the very low hydrodynamic energy back-reef environments of Tethys, but is absent from the American province. The American Borelis has aligned septula and pre-septal passages, but they are generally smaller in size than those of the Tethyan Borelis. American Borelis and Tethyan Borelis are probably examples of homoplasy, as they do not co-exist, and are in fact separated by 6.6 Ma in the geological column.
The Rhapydioninidae were common and mainly endemic to the Late Cretaceous low-latitude shallow-water reefal and lagoonal settings of the American province. All of them, however, went extinct at the end of the Cretaceous. The rhapydioninids of the Paleogene are also distinct from those described from the Cretaceous of the Tethyan province, and are only found in the American province. The genus Raadshoovenia (R. guatemalensis van den Bold) was originally established by van den Bold [ 91] from the Eocene of Guatemala, however, as discussed above, it is different from the Raadshoovenia misidentified in the Tethys, which are in fact Murciella. As is also discussed above, the appearance of Raadshoovenia in the Paleocene–Eocene of the American province is therefore yet another example of homoplasy, where similar characters arose by convergent evolution. True Raadshoovenia has been reported from Mexico [ 92, 93] and Guatemala [ 18, 51, 57]. There are no Paleocene records of Raadshoovenia from the Tethyan province.
The American Raadshoovenia gave rise to Neomurciella, which made its first appearance in the Selandian of the American province (PZ P3a [ 18, 94]). Neomurciella show a crosswise development of the central and marginal chamberlets, as was found in the Campanian–Maastrichtian Murciella gr. M. renzi Fleury (see [ 18, 65, 50]), but this reappearance is 4.4 Ma later than the disappearance of Murciella. Neomurciella survived the Paleocene–Eocene boundary only to disappear within the Early Eocene.
The American Fabulariidae are represented by members of the genus Fabularia. This genus evolved for the first time in the American province from the small, primitive form Miliola in the Early Eocene (Ypresian, PZ P5b) by developing an involute, biloculine last stage after the early quinqueloculine stage, and thickened vertical partitions, which subdivide the chambers into elongated chamberlets with two tiers in the outer whorls. The two chambers per whorl is a key characteristic of the megalospheric forms (see [ 1]). The fabulariids were represented in the American province by endemic species, such as F. vaughani Cole, F. cassis, F. colei and F. verseyi. These species are only found in the Caribbean region [ 32, 95, 96] from the Early to Middle Eocene (PZ P6–P12a). In the Middle Eocene, Lutetian (PZ P10), the biloculine Fabularia ( Fig. 7) gave rise to Pseudofabularia ( Plate 3, l) by developing alveolinid-like pre-septal passages [ 97]. The Bartonian–Priabonian fabulariid assemblages manifested gigantism, however, they all disappear at the end of the Eocene, except for Fabularia verseyi, which survived the Eocene–Oligocene extinction, only to die out in the Rupelian [ 98, 99].
The Tethyan Province
Although the Tethyan province was still the hotspot for Alveolinidae diversity, the Paleocene forms evolved independently from their Late Cretaceous relatives ( Fig. 8). In the Tethyan province, they re-appeared during the warm Late Paleocene (Thanetian), where they colonised reefal and backreef environments. During the Eocene, they became large and abundant in forereef environments, only to largely disappear by the end of the Eocene.
Paleogene alveolinids presumably evolved, as did the Cretaceous forms, from Pseudonummuloculina (see [ 15, 67]). They subsequently develop lineages that are spherical to fusiform in shape, and planispiral in the adult part, exhibiting morphological convergence (homoplasy) with the fusiform/globular alveolinids of the mid-Cretaceous (e.g. the Praealveolina group). A considerable gap, however, of about 20 Ma exists in the fossil record between the Alveolinidae of the Paleocene and the mid-Cretaceous Praealveolina group.
The evolutionary lineages of the Paleogene alveolinids show an incremental increase in the length of the test from pole to pole, from globular through ovoid to elongate, and spindle-shaped. The oldest Paleogene form, Glomalveolina ( Plate 3, i–k), with a streptoloculine origin and with a fixed axial coiling throughout its ontogeny, is considered the ancestor of Alveolina. Glomalveolina is small, globular and non-flosculinised, with a spherical proloculus in a streptospiral coil and a later planispiral stage. The streptospiral coil still exists in the microspheric forms of Alveolina. Alveolina ( Plate 4, a–j; Plate 5, a–j; Plate 6, a) is planispiral–fusiform with a single tier of chamberlets in each chamber and, unlike Glomalveolina, shows considerable thickening of the basal layer in the equatorial zone (flosculinisation). Pre-septal and post-septal passages are present in both Glomalveolina and Alveolina.
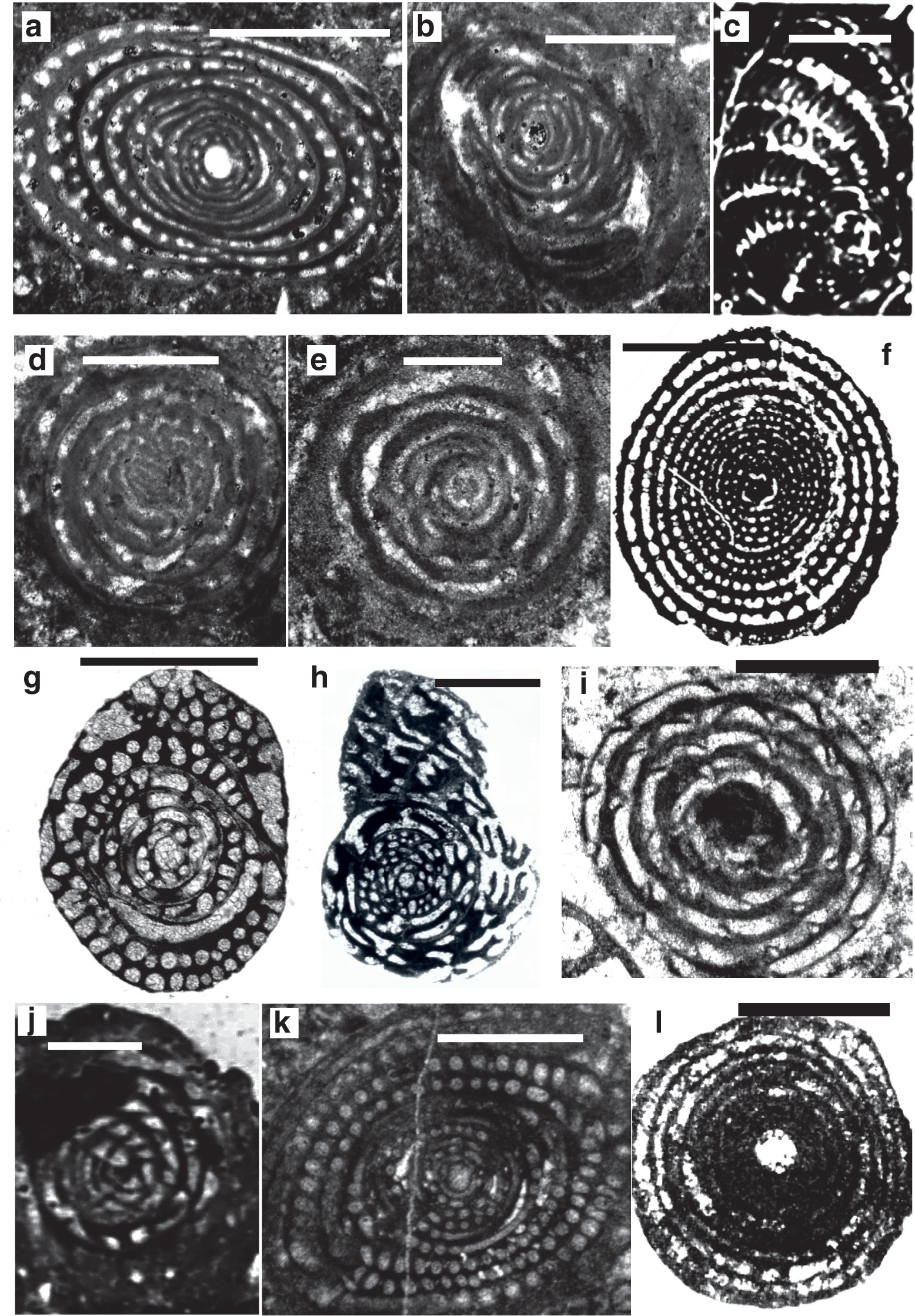
Scale bars: a–j = 1 mm.
a–c. Alveolina globosa (Leymerie), Ypresian, Eocene, Laki Limestone (Laki Formation), Sakesar Peak in the Salt Range, Pakistan, a) UCL coll.; b) NHM Davies coll.; c) Toraja Formation (Sulawesi), SEA coll., Royal Holloway.
d–f. Alveolina oblonga d’Orbigny, Early Eocene, d–e) Dunghan, Siah Koh, UCL coll; f) Chaussy, Val-d’Oise, France, UCL coll.
g. Alveolina leupoldi Hottinger, Early Eocene, Qumiba section, Tingri, Tibet, Nanjing University.
h. Alveolina elliptica (Sowerby), Eocene, Afghanistan, NHM Skinner coll. P7404.
i. Alveolina corbarica Hottinger, Early Eocene, Longjiang section, Tibet, Nanjing.
j. Alveolina daniensis Drobne, Early Eocene, Ladakh region, India. Sagar Damania, Indian Institute of Technology coll.
Scale bars: a–j = 1 mm.
a. Alveolina vredenburgi Davies 1937 (= Alveolina cucumiformis Hottinger), topotype, axial section, Late Paleocene, Aquitaine, France, UCL coll.
b. Alveolina pasticillata Schwager, Early Eocene, Ladakh region, India, Sagar Damania, Indian Institute of Technology coll.
c–d. Alveolina stipes Hottinger, Middle Eocene, Ainsa, Spain, UCL coll.
e. Alveolina solida Hottinger, Early Eocene, Ladakh region, India, Sagar Damania, Indian Institute of Technology coll.
f. Alveolina leupoldi, Hottinger, Early Eocene, Coustouge, France, UCL coll.
g. Alveolina palermitana Hottinger, Middle Eocene, Middle Khirthar, Pakistan, UCL coll.
h. Alveolina vredenburgi Davies, topotype, equatorial section, Late Paleocene, Aquitaine, France, UCL coll.
i–j. Alveolina subpyrenaica Leymerie, Early Eocene, Zagros, Iran, UCL coll.
Scale bars: a–j = 1 mm.
a–b. Alveolina laxa Fottinger, Early Eocene, Egypt, UCL coll.
c. Globoreticulina iranica Rahaghi, figured by Hottinger [ 67], Middle Eocene, Shiraz, Iran.
d. Borelis pygmaeus Hanzawa, Oligocene, Borneo, 65/9 Loc.205, UCL coll.
e. Borelis vonderschmitti (Schweighauser), Late Eocene, Syria, UCL coll.
f. Borelis curdica (Reichel), Miocene, Turkey, NHM coll.
g. Borelis melo (Fichtel and Moll), Middle Miocene, Turkey, NHM P49087.
h. Borelis haueri (d’Orbigny), Middle Miocene, Baden-Brickyard, Baden, Australia, UCL coll.
i. Lacazinella wichmanni (Schlumberger), Late Eocene, Indonesia, UCL coll.
j. Fabularia discolithus Defrance, Eocene, calcaire grosier de Rennes, France, NHM Brady coll. P41603.
In the Eocene the amount of flosculinisation varies in different species of Alveolina, and might be considered to be of specific value only. Alveolina evolve into larger forms through the Eocene, and more than 90 species of the genus Alveolina have been reported in the Mediterranean [ 8]. Many of the species reached gigantic sizes (up to 20 mm long [ 100]) before the end of the Middle Eocene, where they become essential members of Eocene carbonate fossil assemblages. Alveolina species, however, became smaller again before completely disappearing at the end of Eocene (see [ 1]).
In the Eocene Alveolina gave rise to new genera, such as Globoreticulina ( Plate 6, c) and Malatyna, which exhibited similar trends of evolution to the Cretaceous Subalveolina and Praebullalveolina. They developed streptospiral nepionts in both generations and a planispiral-involute chamber arrangement in the adult stage of growth [ 67, 101]. Both these genera became extinct at the end of the Lutetian.
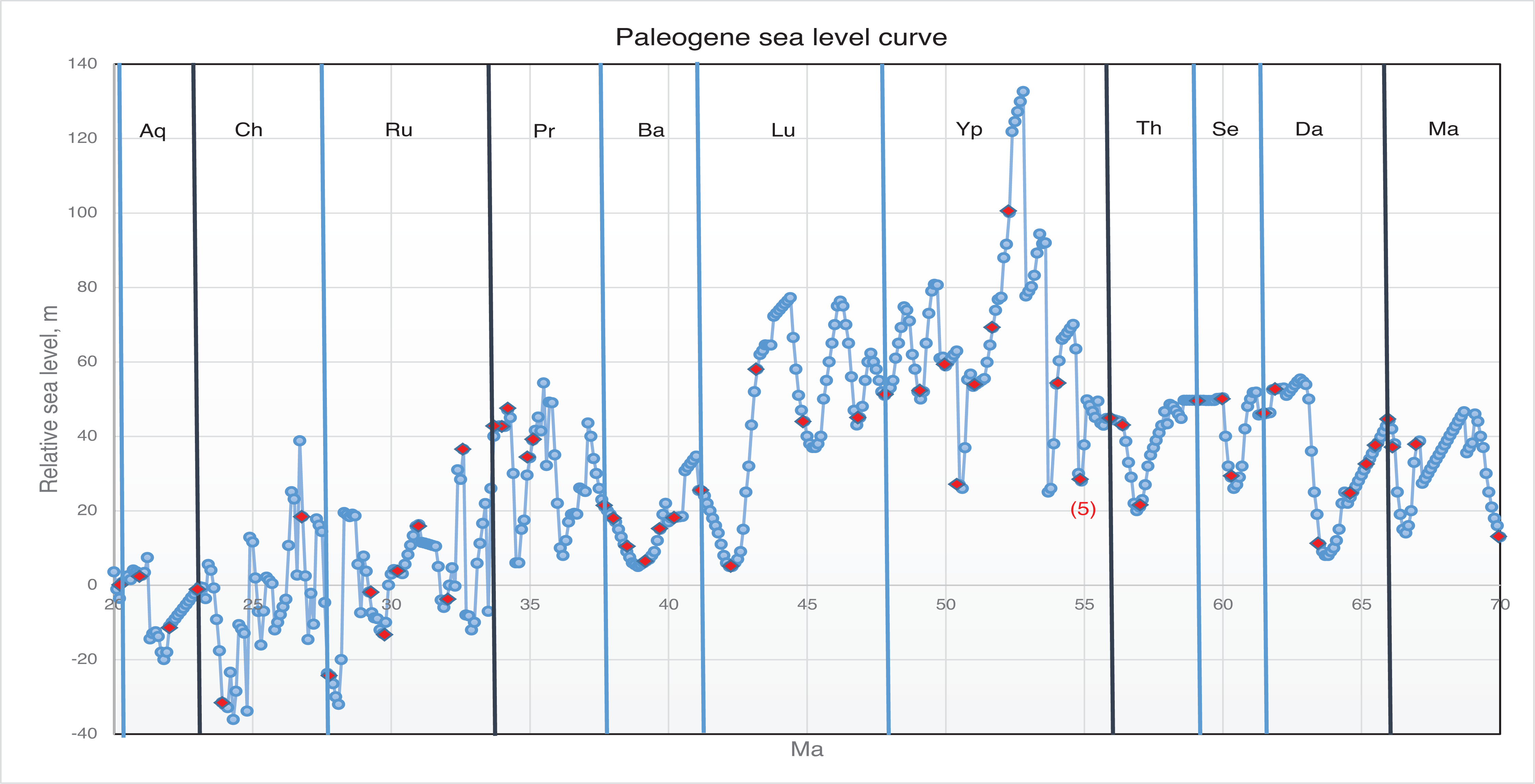
Paleoclimatic evidence shows a trend of cooling during late Middle Eocene and at the Eocene–Oligocene (E–O) boundary ([ 102]; Fig. 13). At the Middle to Late Eocene boundary (37.8 Ma) major changes in global climate and ocean circulation [ 102] were likely responsible for the disappearance of the gigantic alveolinid species seen at the end of the Bartonian (see [ 1]). The E–O boundary cooling period followed the onset of the Late Eocene rapid sea-level changes, which led to the disappearance of vast carbonate platform and lagoonal environments, and the final extinction of Alveolina, and indeed of 85% of all Alveolinoidea. The move into an ‘ice house’ climate may have been triggered at this time by the opening of the Tasmanian gateway [ 103].
The only alveolinid survivors of the E–O cooling event were forms related to Borelis ( Plate 6, d–h), which is in fact an extant alveolinid, and the only one to survive into the Neogene. The appearance of Borelis in the Tethyan province in the Bartonian was 6.6 Ma years after the last recorded Borelis species in the Paleogene of the American province. Given the magnitude of this gap in the stratigraphic column, we suggest that it is unlikely that this appearance in Tethys was the result of a migration event. We suggest that it is more probable that this appearance in Tethys is yet another example of homoplasy, where re-evolution from a primitive form gave rise to a form in Tethys that was generically similar to the extinct American form, and that it filled the Tethyan niche previously occupied by Glomalveolina.
In the Tethyan Eocene, Borelis is represented by the spheroidal B. vonderschmitti (Schweighauser), which was widespread throughout the Bartonian and Priabonian of Western Tethys [ 104, 105]. Borelis is common in deposits laid down in Early Oligocene, low-latitude, shallow-marine environments, and differs from Alveolina in having only a pre-septal passage and a secondary small tier of chamberlets, which alternate with the larger ones producing ‘Y’ shaped septa in the axial section [ 106]. In the Oligocene, the sub-spheroidal, small (0.5–1.5 mm in diameter and 0.4–1.2 mm long), tightly coiled Borelis inflata (a form with no Y-shaped septula, see [ 105]) is also recorded from the Oligocene to the Tortonian of the Western Tethys [ 107– 110].
In the Early Oligocene (from about 33.9 Ma), the Drake Passage opened and there was further climatic cooling and ice volume increase ([ 102]; see Fig. 13). Borelis species, which survived the E–O boundary, became adapted to globally cooler environments, while others migrated to the warmer Tethys, such as B. inflata.
The Oligocene continue to witness an evolutionary trend of small alveolinids in the Mediterranean, such as Bullalveolina. This form evolved from being streptospiral to having a planispiral quinqueloculine test, but unlike the extant Borelis, Bullalveolina disappeared at the end of the Oligocene. Generally, however, there were very few notable extinctions of LBF at the end of the Oligocene (see [ 1]). This stratigraphic boundary is probably connected to plate tectonic events, such the development of the Alpine–Himalayan orogeny, which were more gradual events and did not seem to trigger major extinctions, or to gradual changes in climate caused by the growing thermal isolation of Antarctica as Australia drifted northwards [ 102].
As in the Cretaceous, the Paleogene Fabulariidae (mainly the lacazinoform genera, Lacazina, Lacazinella) are directly derived from the small primitive Idalina which survived across the K–P boundary (see Fig. 8). Further to the ranges listed in BouDagher-Fadel [ 1], it now seems that the Fabulariidae re-appeared, independently from their Cretaceous counterparts. In doing so, they developed morphologically convergent, homoplasic lineages, which has led to these lineages being given the same generic names as their Cretaceous counterparts. However, we again contend that the considerable stratigraphic gap between their reappearance in the Middle–Late Paleocene and the end Cretaceous forms excludes any chance of a direct phylogenetic relationship, other than via a homoplasic repeated evolution from the same Idalina common ancestor. There are notable differences between Cretaceous and Paleogene genera, so for example, all Paleogene representatives of Pseudolacazina are distinguished from the Cretaceous forms in having lower in shape chambers, subdivided by continuous chamber partitions (septula) (see [ 15, 111]).
In the Paleocene, Periloculina and Pseudolacazina echoed the Cretaceous forms, but they uniquely gave rise to Paleogene Lacazina and Lacazinella by gradual ontogenetic transformation from pluri- to unilocular, completely overlapping growth. The two chambers per whorl is a key characteristic of the megalospheric forms in the fabulariid, however, in Lacazina and Lacazinella, the microspheric tests have a small initial biloculine stage, followed by uniloculine growth, while their megalospheric tests only show uniloculine development, with a large proloculus. The basal layer of Pseudolacazina bears radial pillars supporting the chamber roof that evolve into low longitudinal septula, which do not reach the chamber floor in Lacazinella, to continuous septula supporting the external chamber wall in Lacazina [ 1, 112].
The re-appeared Lacazina is first recorded in Tethys from the Selandian (PZ P4a), while Lacazinella, having completely overlapping chambers, is rare in the Tethyan province and has not been found in Europe, but is only recorded from Oman and other parts of the Middle East [ 113].
On the other hand, the American Fabularia spp., which first appeared in the American province in PZ P5b [ 96], appears in Tethys ( Plate 6, j) in the PZ P6b [ 32, 111, 114]. This west to east trans-Atlantic migration coincides with one of two global sea-level low stand in the early Ypresian (migration 5 in Figs 8, 13 and 14).
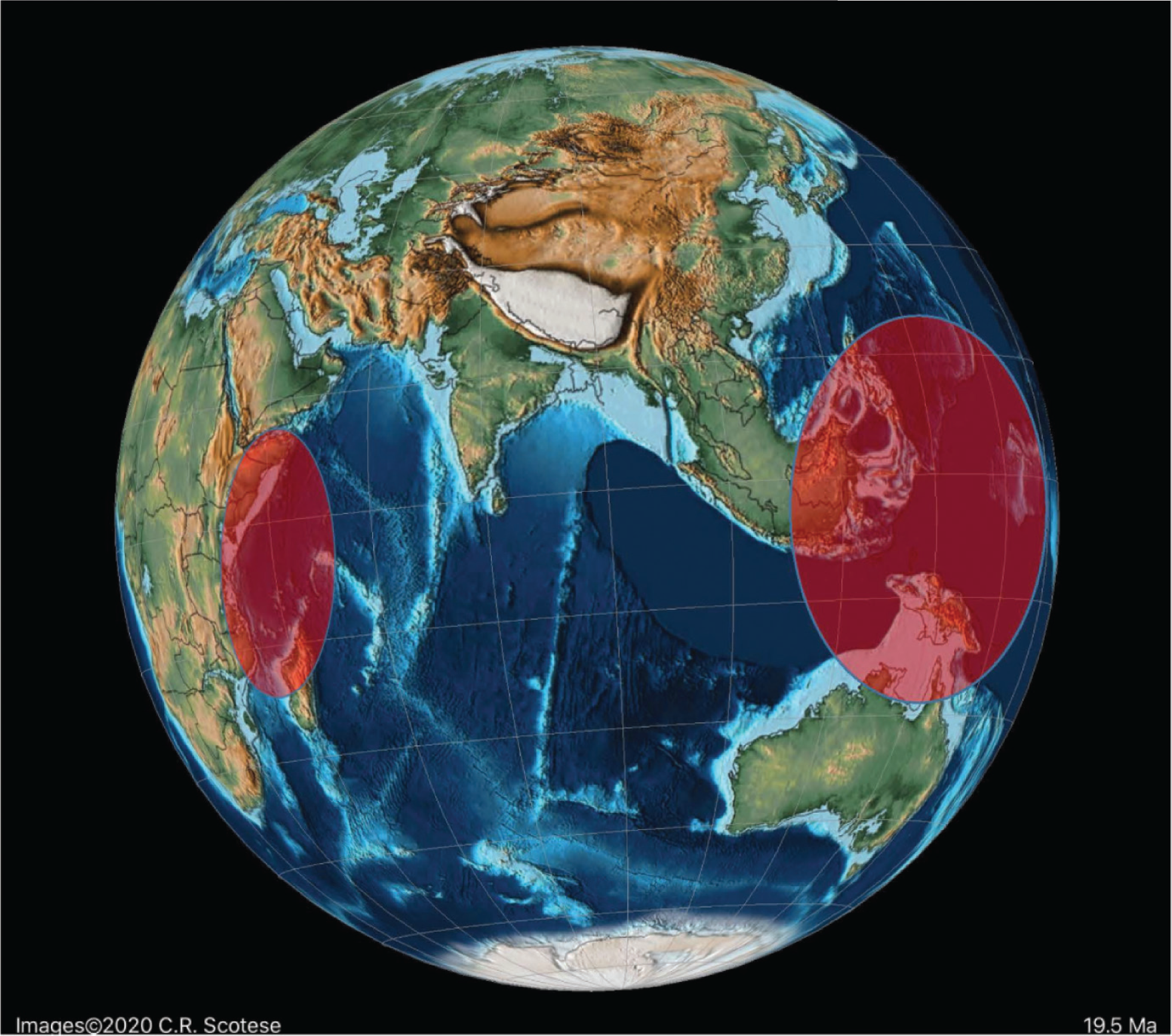
The Indo-Pacific Province
We found in our earlier studies of Paleogene rotaliids that migration from Tethys to the Indo-Pacific commonly occurred. Likewise, here we find examples of eastward migration in the Eocene of some Alveolinidae into the Indo-Pacific province after their first appearance in Tethys ( Fig. 14). Thus, the first species of Alveolina appears in the late Ypresian (PZ P9; migration 6 on Fig. 15) in the Indo-Pacific province, compared with its occurrence at the base of Thanetian (PZ P4b) in the Tethys. The Indo-Pacific alveolinids of the Paleogene remained of lower diversity than those of their Tethyan ancestors. Their sizes were smaller (ranging between 7 and 10 mm [ 23]) and they showed less internal morphological variation. Only a few species have been reported from Indonesia, and many of them are of the Alveolina oblonga group of Hottinger [ 8]. The Indo-Pacific alveolinids range from the latest Ypresian (P9) to Bartonian [ 115]. Other species reported from the Indo-Pacific province include Alveolina timorensis Verbeek and Alveolina ovicula Nuttall [ 116].
Borelis, which first appeared in the Bartonian in Tethyan province, migrated towards the Indo-Pacific through the Tethyan corridor ( Fig. 17), but did not appear in the Indo-Pacific province (migration event 9 in Fig. 8 [ 1, 115, 117]) before the Priabonian (PZ P15b; letter stage, Tb). For example, the Tethyan Borelis vonderschmitti (Schweighauser), which was widespread throughout the Middle–Late Eocene in the Western Tethys [ 104], appeared in the Late Eocene of the Indo-Pacific province [ 118]. The Tethyan Oligocene species of B. inflata are recorded also from the Oligocene–Miocene of Sarawak (Borneo) in the Indo-Pacific area [ 118, 119]. A gradual stratigraphic succession of Indo-Pacific species marked its very slow evolution throughout the Oligocene.
Some Tethyan Fabulariidae forms also migrated into the Indo-Pacific province [ 120– 122], where they are represented by Lacazina and Lacazinella. Lacazina reached the Indo-Pacific (migration 8 in Fig. 8) in the Bartonian (late P12a) and settled there by the late Eocene. Lacazina representatives are confined to the northern margin of the Indo-Pacific realm and are found only on an isolated platform of Moluccas [ 31]. Lacazinella, restricted to the southern part of the Tethyan province in the early Eocene (Somalia, Oman), first appears in the Lutetian in the Indo-Pacific province (migration 7 in Fig. 8) and in the Late Eocene (Papua New Guinea). This genus is recorded from Mindoro and Timor, and on remnants of the Australian craton in Indonesia [ 122]. Lacazinella did not survive the E–O extinction. Neolacazopsis, the representative of the Fabulariidae in the Middle to Late Eocene of Japan, evolved from Lacazina by developing turtleneck, bottle-like chamberlets and alveolar inner wall instead of arcuate chamberlets [ 123].
The Neogene Alveolinoidea
Only Alveolinidae survived into the Neogene. The eastward migration towards the Indo-Pacific province along the Tethyan corridor previously seen in the Paleogene continued into the early Neogene. As species became geographically isolated, the alveolinid distribution showed a distinct provincialism. They colonised new areas in the Indo-Pacific and evolved similar but distinct parallel lineages, taking advantage of empty niches and optimal conditions.
The Tethyan/Mediterranean Province
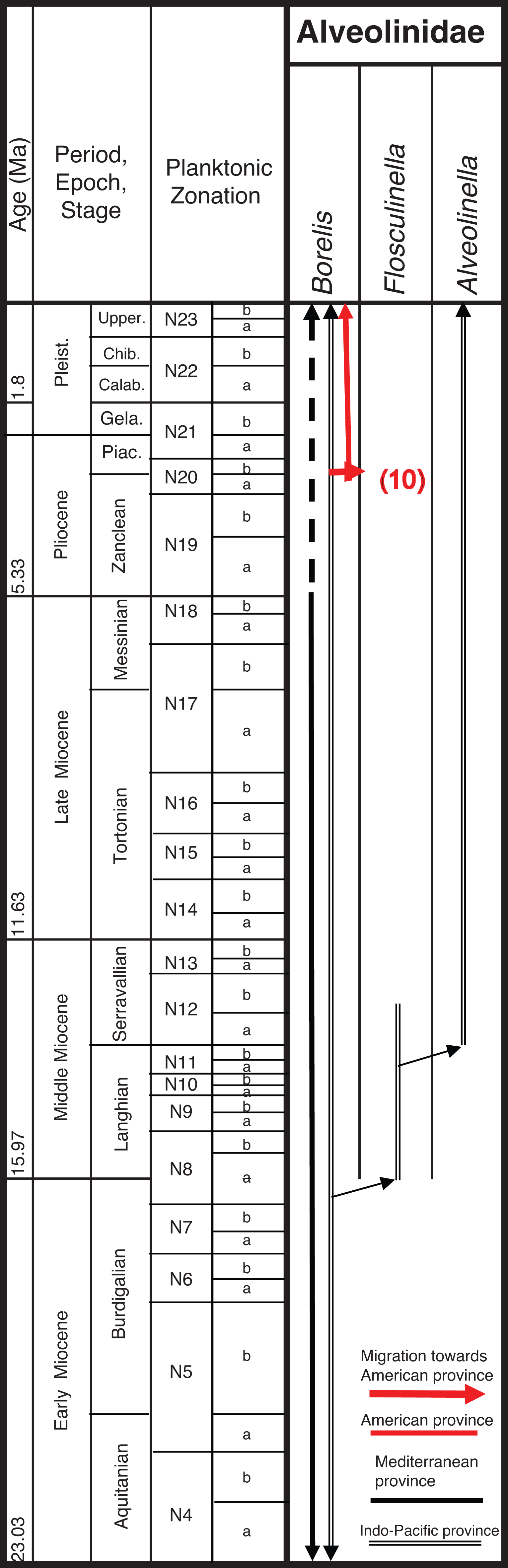
In the Miocene, the Tethyan alveolinids were represented by species, such as B. inflata, Borelis melo and Borelis curdica. Borelis inflata is recorded as ranging from the Oligocene to the Miocene (Tortonian) of the Western Tethys [ 107, 110, 124], and Zakynthos in Greece [ 108, 109]. Borelis melo with a spheroidal shape and relatively large proloculus (60–65 μm) has been reported from the Aquitanian to Messinian in the Tethyan province [ 105, 106, 125– 127]. According to Bassi et al. [ 105], in B. melo the Y-shaped septula occur only in the adult growth stage, while in B. curdica, the proloculus is smaller (30–50 μm) and the Y-shaped septula occur in all whorls. These authors also postulated that all reliable records of B. melo and B. curdica are only from Western Tethys, and B. curdica is restricted to the Tethyan province. Borelis melo ranges from the Aquitanian to the earliest Messinian, while B. curdica ranges from the Burdigalian to the Tortonian [ 105, 106, 126, 128– 132]. For a comprehensive study and complete references to Borelis species, the reader is referred to Jones et al. [ 133] and Bassi et al. [ 105].
Tethyan alveolinid species became isolated from the Indo-Pacific by the final eastern closure of the Mediterranean basin ( Fig. 18), and finally disappeared from the Mediterranean during the Messinian salinity crisis. However, living Borelis species have been reported from the shallow (less than 40 m) continental shelf off Israel [ 134]. Their re-occurrence in the Eastern Mediterranean with other LBF is attributed by Hyams et al. [ 134] either to migration from the Atlantic during warm periods of the Pleistocene or Holocene, or to modern migration from the Atlantic, or to modern (Lessepsian) migration from the Red Sea via the Suez Canal. Since the opening of the Suez Canal in 1869, the flow of Lessepsian species (i.e. Indo-Pacific species introduced into the Mediterranean Sea via the canal) has been continuous [ 135]. We suggest that a Lessepsian migration is the most likely way that Borelis has recently been introduced to the Eastern Mediterranean, as it is not found in the Western Mediterranean, which would be expected had the migration been from the Atlantic. Furthermore, Hyams et al. [ 134] figured solid, sub-ellipsoidal specimens morphologically similar to the Indo-Pacific Borelis schlumbergeri (Reichel). The Lessepsian origin of this finding is further supported by the fact that B. schlumbergeri (Reichel) is to be found in the Northern Bay of Safaga, in the Red sea, close to the southern entrance to the Suez Canal [ 136].
The Indo-Pacific Province
In contrast to the apparent isolation of Tethys forms from the Americas in the Middle and Late Paleogene, migration towards the Indo-Pacific continued in the Oligocene and Early Miocene through the Tethyan seaway (see [ 3– 6]). In the late Langhian–early Serravallian (13–14 Ma), a time of several regressions, the short-lived marine reconnection between the Mediterranean and Indian Ocean again closed [ 137]. This final closure brought all migration towards the Indo-Pacific province to an end, and coincided with the onset of a global cooling period. By the time the East Antarctic Ice Sheet was established (12 Ma), the forereef LBF such as the miogypsinids and lepidocyclinids had become extinct or very rare in the Indo-Pacific sub-province [ 1], while the isolated backreef LBF in the Indo-Pacific developed new survival characters. The small primitive Borelis in Middle to Late Miocene shallow-water settings became rare and was gradually replaced by forms with more evolved characters [ 1, 27], such as Flosculinella ( Plate 7, a–b) with double rows of chamberlets on the floor of each chamber, and the elongated fusiform Alveolinella with several rows of chamberlets in axial section. The superposed regular layers of chamberlets separated by floors in Alveolinella ( Plate 7, c–g) are analogous structures found in the mid-Cretaceous Praealveolina, and are different from the irregular, tubular, supplementary chamberlets present in the thickened basal layers of the Paleogene Alveolina [ 67]. They re-appear, through convergent evolution (homoplasy), millions of years after their earlier extinction, as they are selected for by the changed ecological conditions developed in the Middle Miocene.
Scale bars: a–g = 1 mm.
a. Flosculinella globulosa (Rutten), Early Miocene (lowermost Burdigalian), Indonesia, UCL coll.
b. Flosculinella bontangensis (Rutten), late Early Miocene (Burdigalian, lower Tf1), Borneo, UCL coll.
c. Alveolinella praequoyi Wonders and Adams, holotype, early Middle Miocene (upper Tf1–Tf2), Darai Limestone, Papua New Guinea, NHM P52658.
d. Alveolinella fennemai Checchia-Rispoli, late Early Miocene (Burdigalian), Borneo, UCL coll.
e–g. Alveolinella quoyi (d Orbigny), Holocene, e) Sekau, formation SEA coll., Royal Holloway; f) Port Moresby, Coral Sea, New Guinea, UCL coll; g) Pacific, UCL coll.
Over the Neogene Period, Indo-Pacific alveolinids underwent a very slow evolution, but notably include Borelis pygmaeus (which persists to the top of the Te stage [ 1, 27]). This species is recorded from Oligocene to Lower Miocene deposits [ 23, 27, 118, 119], but is suddenly replaced by a more advanced form Flosculinella bontangensis ( Plate 7, b), with an incremental increase in the length of the test from pole to pole, changing from globular, to ovoid, to elongated spindle-shaped, and with an equally incremental increase in the number of secondary chamberlets (‘mansardes’) from zero in Borelis through to one in Flosculinella to several rows of chamberlets in Alveolinella. Parallel lineages have each developed with the acquisition of an additional row of chamberlets in the Borelis–Flosculinella lineage. This must have occurred at least twice during Te–Tf interval (Early–Middle Miocene). An example of one of these lineages is seen with the appearance of Flosculinella reicheli within the Early Miocene (upper Te) and of F. bontangensis ( Plate 7, b) in the early Middle Miocene (just at the top of the Te stage). The latter gave rise to Alveolinella praequoyi ( Plate 7, c) in the Serravallian, at the base of Tf2 stage. Alveolinella praequoyi has early whorls akin to F. bontangensis, but in the latter the chamberlets of each whorl are covered by at least two layers of smaller chamberlets. In Alveolinella quoyi ( Fig. 9; Plate 7, e–g), in the Tf3 stage, all of the whorls have a multiple layer of chamberlets. Flosculinella bontangensis grades into forms of Alveolinella at about the same time as the onset of the global cooling. Alveolinella today appear to be confined to normal shallow marine (down to depths of 80 m), clear, well-oxygenated, tropical and subtropical waters [ 39, 138].
Different parallel lineages of Borelis spp. evolved in the Indo-Pacific area. Those with Y-shaped septula such as B. pygmaeus are recorded in the Indo-Pacific from the Rupelian to latest Burdigalian (uppermost upper Te [ 27]), while other lineages without the Y-shaped septula in the juvenile stage, such as B. schlumbergeri, are recorded from the Tortonian to Holocene [ 105, 139, 140].
Bassi et al. [ 105] assigned the Indo-Pacific Oligocene to Early Miocene species of Borelis primitiva (described from an Upper Oligocene limestone from Eniwetok [ 141]), Borelis globose, Borelis boninensis (described from the Rupelian of east Mindanao and from the the Bonin Islands [ 118, 142]), and the specimen of B. melo (reported by BouDagher-Fadel [ 1] from the Serravallian of Australia), to Borelis pulchra. They based their conclusion on the absence of Y-shaped septula and the proloculus size of B. pulchra. In our opinion, however, these species all have specific characteristics and are separated by time and space from B. pulchra. We suggest, therefore, that they should still be treated as separate species.
Borelis inflata, which first appeared in the Oligocene of Tethys, migrated rapidly into the Indo-Pacific province, and has been described from the Oligocene to Lower Miocene of Sarawak (Borneo) in the Indo-Pacific area [ 118, 119].
The American Province
The alveolinids were absent from the Neogene of the American province before the early Piacenzian closure of the Central America seaway ( Figs 13 and 16). Today, they are represented by B. pulchra (d’Orbigny), which is recorded from present-day sands of Cuba, Florida and from Jarvis Island [ 105, 138, 143– 145].
There are no records for this species from the Mediterranean, nor would we contend from the Indo-Pacific (see above). Bassi et al. [ 105], however, suggest that Indo-Pacific forms are synonyms of B. pulchra and proposed that there was a migration eastwards from the Indo-Pacific across the Pacific and through the Central America Seaway, before its closure in the Pliocene ( Fig. 13). However, despite being strong advocates for the dispersal of LBFs by trans-oceanic migration, because in our opinion the Pacific species are distinct from the American B. pulchra, we favour the suggestion that B. pulchra is another example of homoplasy, just as we suggest that the Middle Eocene Tethyan origin of Borelis was distinct from that of the early Paleogene American forms.
Discussion
The synthesis described here of the alveolinoids, over an expanded, globally comprehensive geographic area and stratigraphic range, has enabled a more coherent view of their phylogenetic and palaeogeographic evolution to be established, and provides an update to the description presented in BouDagher-Fadel [ 1]. Two significant characteristics of their evolution and development will be discussed below in further detail, namely those of homoplasy, and of provincialism punctuated by trans-oceanic migration.
The traits of convergent evolution exhibited by the alveolinoids fall into two distinct types: those in which gross external morphology of forms echo the shape of previous LBF forms, and those in which the internal test morphology is reproduced in forms that are not directly consanguineous. As noted above, an extreme example of ecologically driven convergent evolution involving forms from different orders is exhibited in the external appearance of alveolinoids that closely resemble the planispiral, fusiform fusulinides of the late Paleozoic.
More confusingly from the taxonomic and phylogenetic viewpoint, however, are the second type of homoplasy, where due to a predisposition to repeated genetic mutations, forms with the same or similar generic characteristics repeatedly appear within a family over time. When these characteristics are similar but not identical, they can lead to the misidentification of forms, as for example, in the rhapydioninids, where some Cretaceous Tethyan Murciella have been misidentified as Raadshoovenia. As noted above, true Raadshoovenia appeared only in the Paleogene of the American, and can be characterised by having a streptospiral nepiont in both megalospheric and microspheric generations, and by being smaller than Murciella.
Another example of homoplasy is one that gives rise to very similar forms within the same family, but which are separated by a larger stratigraphic interval. So, for example, following their Cretaceous predecessors, Paleogene alveolinids re-appeared from Pseudonummuloculina, giving rise to the Alveolina lineage. These Paleogene forms are morphologically convergent with the fusiform/globular alveolinids of the mid-Cretaceous characterised by the Praealveolina group. There are generic differences to the detailed test structure of these two lineages, as well as a considerable gap of about 20 Ma in the fossil record, so there is no doubt that they represent distinct, homoplasic stages of evolution within the Alveolinidae. The same is not true, however, for the Late Cretaceous and Early Paleogene fabulariids. Here, the Paleocene Periloculina lineage (including Pseudolacazina and Lacazina) echoed the Cretaceous forms so closely that they are generically indistinguishable. There is however a gap of over 6 Ma between the K–P boundary and the first Paleogene occurrence of Periloculina in the Selandian. These Paleogene genera could be considered to be Lazarus taxa, which Wignall and Benton [ 146] explain thus, ‘At times of biotic crisis many taxa go extinct, but others only temporarily disappeared from the fossil record, often for intervals measured in millions of years, before reappearing unchanged.’ Wignall and Benton [ 146] suggest that the reappearance of Lazarus taxa in the fossil record probably reflects the rebound after a period of extreme rarity during the aftermath of a global extinction. Here, however, we prefer another explanation, namely that, as with the Alveolina lineage, the Paleogene Periloculina lineage is an example of homoplasic re-evolution from a small, simple form that survived the K–P extinction. However, unlike Alveolina we suggest that, for the Paleogene Periloculina lineage, the homoplasy produced forms so close to the previous Cretaceous forms as to be generically indistinguishable.
A further example of a form that could be a Lazarus taxa or an example of homoplasy, is given by Borelis. The American Borelis is reported from the Middle Paleocene to the Early Eocene (PZ P–P9), while the first occurrence of the Tethyan Borelis is in the Bartonian of Western Tethys. These occurrences are separated by 6.6 Ma in the geological column, and by an ocean. American Borelis has aligned septula and pre-septal passages, and they are in general smaller in size than those of the Tethyan Borelis. This, and the challenge of the concept of ‘the rebound after a period of extreme rarity’ of a form into a province displaced from its original realm, leads us to propose that American Borelis and Tethyan Borelis are likely also to be examples of homoplasy. This potential tension between the concepts of Lazarus taxa and of homoplasy is worthy of further exploration elsewhere.
We now turn to the second notable feature of the development of the Alveolinoidea, namely their distinct global pattern of provincialism and the episodes of trans-oceanic migration, similar to behaviour previously observed in other LBFs (e.g. [ 3– 6, 147, 148]). The three main alveolinoid provinces were those of the Americas, Tethys and the Indo-Pacific. We have previously demonstrated that the Cretaceous agglutinated orbitolinids originated in Tethys, but underwent periodic westward trans-oceanic migration to the Americas during periods of global sea-level low stands [ 2]. In this study, we have confirmed that the Tethyan province also remained the palaeogeographic centre for the porcelaneous alveolinids, and that they inhabited similar ecological habitats throughout the Cretaceous and Paleogene. It has been inferred here for the first time that a westward palaeogeographic migration of some alveolinid genera also occurred in the mid-Cretaceous from the Tethys to the Americas (migrations 1–3, Fig. 11), while the Late Cretaceous alveolinids remained mainly isolated (migration 4, Fig. 11). On the other hand, in the Paleogene we saw limited eastward migration from the American province towards Tethys ( Fabularia in the Ypresian, PZ P6b; migration 5, Fig. 15). On the other hand, migrations from Tethys towards the Indo-Pacific region continued till the closure of the Tethyan corridor and the isolation of the Mediterranean in the Serravallian (migrations 6–9, Fig. 8). As noted, we have shown that most cosmopolitan alveolinoids appeared in Tethys before migrating to other provinces. Likewise, we have seen that once established in the American and Indo-Pacific provinces, local provincial forms evolved, indicating that they were periodically effectively isolated from the Tethyan province. These characteristics have been observed in previous studies of Mesozoic [ 2] and Cenozoic LBF, specifically the lepidocyclinids [ 3], the miogypsinids [ 4], the nummulitoids [ 5, 149] and the orthophragminids [ 6, 150]. The periods of migration from one province to another were often followed by subsequent isolation and development of local provincial lineages. These migrations were often associated with major sea-level regressions, while the subsequent provincial isolation coincided with global sea-level transgressions. This study has, therefore, further tested the migration hypothesis highlighted by Pignatti [ 7], and has confirmed the wider application of the ideas initially put forward by BouDagher-Fadel and Price [ 2– 6].
Conclusions
We have resynthesised the literature on the occurrence and distribution of the alveolinoids, and augmented this information with observations on new materials from the Americas, Europe, the Middle East, Tibet and South-East Asia. We have produced a global correlation and integration of the occurrences and stratigraphic ranges of members of the three alveolinoid families, namely the Alveolinidae, the Fabulariidae and the Rhapydioninidae, based upon the planktonic foraminiferal zonal scheme of BouDagher-Fadel [ 1, 24]. In the Cretaceous, alveolinoids are found in the Tethyan and American provinces, while of the later Cenozoic forms, Alveolinidae and Fabulariidae are found in three provinces (Tethys, American and the Indo-Pacific), while the Rhapydioninidae are constrained to the Americas.
Alveolinoids characteristically exhibit convergent evolution, with the repeated re-occurrence of certain morphological features. This homoplasy is predominantly of two types, one which recapitulates the gross external morphology of forms, and one in which the internal test morphology is also reproduced, even to the level of regenerating forms with the same generic characteristics.
We have shown that no alveolinoid survived the K–P extinction, but that members of all three families re-appeared from small miliolids that were resilient enough to pass into the Paleogene. Specifically, we have concluded that:
-
Cretaceous Tethyan Murciella has on occasion been misidentified as a Raadshoovenia, which in fact is an American Paleocene form.
-
Fabularia originated in the Americas at the beginning of the Ypresian, but soon thereafter migrated to Tethys.
-
There is a gap of over 6 Ma between the last occurrence of Periloculina, Pseudolacazina and Lacazina in the Cretaceous and their re-appearance in the Paleogene, and that these taxa are most likely to be examples of homoplasic evolution from a small, simple form that survived the K–P extinction.
-
The 6.6 Ma gap between American Borelis and Tethyan Borelis suggests that they are likely also to be examples of homoplasy.
Finally, our previously developed hypothesis that periodic, sea-level lows enable inter-provincial migration of LBF is strongly supported, and that the behaviour and directions of migration of the alveolinoids is resonant with those of the orbitolinids, the orthophragminids, the nummulitoids, the miogypsinids and the lepidocyclinids. Migration occurred from East to West in the Cretaceous, but was dominated by migration from West to East in the Cenozoic.
Acknowledgements
We would like to thank Prof. Hu Xumian and Jingxin Jiang, Yiwei Xu, Gaoyuan Sun, Wen Lai, PhD students, School of Earth Sciences and Engineering and Engineering, Nanjing University, for their assistance in sampling theTibetian and Iranian sections; Sagar Damania, National Stable Isotope Facility, Indian Institute of Technology; Dr. Peter Scholle, Scholle Petrographic, LLC, Greece; and Prof. Sébastien Castelltort, Department of Earth Sciences, University of Geneva, Switzerland, for their collaboration and in providing us with samples from India, Greece and Spain. We are also grateful to the South East Asia Research Group, Royal Holloway College, and the Natural History Museum for allowing us access to their material. We are indebted to Prof Johann Hohenegger, University of Vienna, and Prof. Pamela Hallock, University of South Florida for carefully and thoroughly reviewing our paper.
Declarations and conflict of interest
The authors declare no conflicts of interest in connection to this article.
Open data and data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
[1] BouDagher-Fadel, MK. (2018a). Evolution and geological significance of larger Benthic Foraminifera. London: UCL Press. p. 704
[2] BouDagher-Fadel, MK; Price, GD. (2019). Global evolution and paleogeographic distribution of mid-Cretaceous orbitolinids. UCL Open – Environment, Available from. DOI: http://dx.doi.org/10.14324/111.444/ucloe.000001 Accessed 26 February 2021
[3] BouDagher-Fadel, MK; Price, GD. (2010). Evolution and paleogeographic distribution of the lepidocyclinids. J Foraminiferal Res 40 : 79–108, Available from. DOI: http://dx.doi.org/10.2113/gsjfr.40.1.79 Accessed 26 February 2021
[4] BouDagher-Fadel, MK; Price, GD. (2013). The phylogenetic and palaeogeographic evolution of the miogypsinid larger benthic foraminifera. J Geol Soc 170 : 185–208, Available from. DOI: http://dx.doi.org/10.1144/jgs2011-149 Accessed 26 February 2021
[5] BouDagher-Fadel, MK; Price, GD. (2014). The phylogenetic and palaeogeographic evolution of the nummulitoid larger benthic foraminifera. Micropaleontology 60 : 483–508.
[6] BouDagher-Fadel, MK; Price, GD. (2017). The paleogeographic evolution of the orthophragminids of the paleogene. J Foraminiferal Res 47 : 337–57, Available from. DOI: http://dx.doi.org/10.2113/gsjfr.47.4.337 Accessed 26 February 2021
[7] Pignatti, J. (2019). Review. Global evolution and paleogeographic distribution of mid-Cretaceous orbitolinids, Available from. DOI: http://dx.doi.org/10.14293/S2199-1006.1.SOR-EARTH.AZZFBB.v1.RVGYLO Accessed 26 February 2021
[8] Hottinger, L. (1960). Uber paleocaene und eocaene Alveolinen. Eclogae Geol Helv 53 : 265–83.
[9] Ciry, R. (1964). À propos de Meandropsina larrazeti Mun.-Chalm., generotype d’un genre nouveau Larrazetia Ciry. Rev de Micropaleontol 6 : 185–95.
[10] Robinson, E. (1968). Chubbina, a new Cretaceous alveolinid genus from Jamaica and Mexico. Palaeontology 11 : 526–34.
[11] Viallard, P. (1973). Recherches sur le cycle alpin dans la Chaine iberique sud-occidentale In: Thèse, Travaux du laboratorie de géologie méditerranéenne. Tolouse: Univérsité Paul Sabatier. p. 445
[12] Fleury, J-J. (1974). Contribution a la connaissance des Rhapydionininae (Foraminiféres, Alveolinidae) crétacés. Geobios 7 (4) : 307–22, Available from. DOI: http://dx.doi.org/10.1016/s0016-6995(74)80013-6 Accessed 26 February 2021
[13] Chaproniere, GCH. (1984). The Neogene larger foraminiferal sequence in the Australian and New Zealand regions, and its relevance to the East Indies letter stage classification. Palaeogeogr Palaeoclimatol Palaeoecol 46 : 25–35, Available from. DOI: http://dx.doi.org/10.1016/0031-0182(84)90023-3 Accessed 26 February 2021
[14] Leppig, U. (1987). Biostratigraphy of larger foraminifera and facies development from the Upper Albian to the Middle Paleocene in the Sierra de Cantabria and the Montes Obarenes (Northwestern Spain). Neues Jahrb Geol Paläontol – Monatshefte Jg 11 : 647–66, Available from. DOI: http://dx.doi.org/10.1127/njgpm/1987/1987/647 Accessed 26 February 2021
[15] Hottinger, L; Drobne, K; Caus, E. (1989). Late cretaceous, larger, complex miliolids (foraminifera) endemic in the Pyrenean faunal province. Facies 21 (1) : 99–133, Available from. DOI: http://dx.doi.org/10.1007/bf02536833 Accessed 26 February 2021
[16] Serra-Kiel, J; Hottinger, L; Caus, E; Drobne, K; Ferrández, C; Jaurhi, AK. (1998). Larger foraminiferal biostratigraphy of the Tethyan Paleocene and Eocene. Bull Soc Geol Fr 169 : 281–99. Accessed 26 February 2021
[17] Vicedo, V; Calonge, A; Caus, E. (2011). Cenomanian rhapydioninids (Foraminiferida): architecture of the shell and stratigraphy. J Foraminiferal Res 41 (1) : 41–52, Available from. DOI: http://dx.doi.org/10.2113/gsjfr.41.1.41 Accessed 26 February 2021
[18] Vicedo, V; Caus, E; Frijia, G. (2013). Late Cretaceous alveolinaceans (larger foraminifera) of the Caribbean palaeobioprovince and their stratigraphic distribution. J Syst Palaeontol 11 : 1–25, Available from. DOI: http://dx.doi.org/10.1080/14772019.2011.637517 Accessed 26 February 2021
[19] Vicedo, V; Berlanga, JA; Serra-Kiel, J. (2014). Paleocene larger foraminifera from the Yucatán Peninsula (SE Mexico). Brest, Carnets de Géologie [ Notebooks on Geology] 14 (4) : 41–68, Available from. DOI: http://dx.doi.org/10.4267/2042/53527 Accessed 26 February 2021
[20] Vicedo V and Piuz, A. (2017). Evolutionary trends and biostratigraphical application of new Cenomanian alveolinoids (Foraminifera) from the Natih Formation of Oman. J Syst Palaeontol 15 : 821–50, Available from. DOI: http://dx.doi.org/10.1080/14772019.2016.1244709 Accessed 26 February 2021
[21] Acar, Ş. (2019). Descriptions, systematics and revisions of the subgenera Alveolina ( Glomalveolina) Hottinger, 1960 and Alveolina ( Alveolina) d’Orbigny, 1826 (Foraminiferida). Bull Min Res Exp 159 : 89–97.
[22] Bozkurt, A; Gormus, M. (2019). Description criteria for eocene alveolinids: examples from inner Western Anatolia. IOP Conf Ser: Earth Environ Sci, : 362. Available from. DOI: http://dx.doi.org/10.1088/1755-1315/362/1/012019 Accessed 26 February 2021
[23] Bakx, LAJ. (1932). De genera Fasciolites en Neoalveolina in het Indo-Pacifische gebied. Verhandelingen van het Koninklijk Nederlandsch Geologisch en Mijnbouwkundig Genootschap voor Nederland en Koloniën 9 : 205–67.
[24] BouDagher-Fadel, MK. (2015). Biostratigraphic and geological significance of planktonic foraminifera. 2nd ed London: UCL Press. p. 320
[25] BouDagher-Fadel, MK. (2018b). Revised diagnostic first and last occurrences of Mesozoic and Cenozoic planktonic foraminifera. UCL Office of the Vice-Provost Research, Professional Papers Series, UCL Press, pp. 1–5.
[26] Gradstein, FM; Ogg, JG; Schmitz, MD; Ogg, GM. (2012). The geologic time scale. Amsterdam, Boston: Elsevier.
[27] Boudagher-Fadel, MK; Banner, F. (1999). Revision of the stratigraphic significance of the Oligocene–Miocene “Letter-Stages”. Rev de Micropaleontol 42 : 93–7, Available from. DOI: http://dx.doi.org/10.1016/S0035-1598(99)90095-8 Accessed 26 February 2021
[28] Schaub, H. (1981). Nummulites et Assilines de la Tethys paléogene. Taxonomy, phylogenèse et biostratigraphie. Schweiz Paläontol Abh 104–106 : 1–236.
[29] Adams, CG. (1973). Some tertiary foraminifera In: Hallam, A (ed.), Atlas of Paleobiogeography. Amsterdam: Elsevier, pp. 453–68.
[30] Hottinger, L. (1973). Selected Paleogene larger foraminifera In: Hallam, A (ed.), Atlas of Palaeobiogeography. Amsterdam: Elsevier, pp. 443–52.
[31] Drobne, K; Hottinger, L. (2004). Larger miliolid foraminifera in time and space. Academie Serbe Sciences et Arts, Bulletin T. CXXVIII; Sciences Naturelles 42 : 83–99.
[32] Drobne, K; Ćosović, V. (2009). Palaeobiogeography of the Late Cretaceous to Paleogene larger miliolids from tropical to subtropical-sea belts (Neotethys to Caribbean). Bull Soc Geol Fr 180 (4) : 317–31, Available from. DOI: http://dx.doi.org/10.2113/gssgfbull.180.4.317 Accessed 26 February 2021
[33] Hottinger, L. (1978). Comparative anatomy of selected foraminiferal shell structures In: Headley, RH, Adams, G G (eds.), Foraminifera III. London: Academic Press, pp. 203–66.
[34] Stanley, GD; Lipps, JH. (2011). Photosymbiosis: the driving force for reef success and failure. Corals and Reefs: Crises, Collapse and Change 17 : 33–59, Available from. DOI: http://dx.doi.org/10.1017/s1089332600002436 Accessed 26 February 2021
[35] Hallock, P. (1985). Why are larger foraminifera large?. Paleobiology 11 : 195–208, Available from. DOI: http://dx.doi.org/10.1017/s0094837300011507
[36] Hottinger, L. (2000). Functional morphology of benthic foraminiferal shells, envelopes of cells beyond measure. Micropaleontology 46 Suppl. 1 : 57–86.
[37] Reichel, M. (1964). Alveolinidae In: Moore, RC (ed.), Treatise on Invertebrate Paleontology, C (Protista 2). Boulder/Lawrence: Geological Society of America/University of Kansas Press, pp. 503–10.
[38] Dilley, FC. (1973). Cretaceous larger foraminifera In: Hallam, A (ed.), Atlas of Palaeobiogeography. Amsterdam: Elsevier, pp. 403–19.
[39] Hohenegger, J. (2000). Coenoclines of larger foraminifera. Micropaleontology 46 Suppl. 1 : 127–51.
[40] Reiss, Z; Hottinger, L. (1984). The Gulf of Aqaba: ecological micropaleontology In: Ecological Studies. Berlin: Springer-Verlag, p. 50 pp. 1–354.
[41] Velić, I. (2007). Stratigraphy and Palaeobiogeography of Mesozoic Benthic Foraminifera of the Karst Dinarides (SE Europe) – PART 1. Geol Croat 60 : 1–113.
[42] Luperto-Sinni, E. (1966). Microfaune del Cretaceo delle Murge baresi. Geol Romana 5 : 117–56.
[43] Calvez, H. (1988). Deux Foraminifères nouveaux de l’Albien calcaire des Pyrénées franco-espagnoles Pseudonummoloculina aurigerica n. gen., n. sp. et Dobrogelina? angulata n. sp. Benthos ‘86. Résumés Abstracts. Genève: Museum d’Histoire Naturelle 2 : 391–99.
[44] Husinec, A; Velić, I; Fuček, L; Vlahović, I; Matičec, D; Oštrić, N; Korbar, T. (2000). Mid Cretaceous orbitolinid (Foraminiferida) record from the islands of Cres and Lošinj (Croatia) and its regional stratigraphic correlation. Cretac Res 21 : 155–71, Available from. DOI: http://dx.doi.org/10.1006/cres.2000.0203 Accessed 26 February 2021
[45] Krobicki, M; Olszewska, O. (2005). Urgonian-type microfossils in exotic pebbles of the Late Cretaceous and Palaeogene gravel stones from the Sromowce and Jarmuta formations (Pieniny Klippen Belt, Polish Carpathians). Studia Geologica Polonica, Kraków 124 : 215–35.
[46] Mancinelli, A; Chiocchini, M. (2006). Cretaceous benthic foraminifers and calcareous algae from Monte Cairo (Southern Latium, Italy). Boll Soc Paleontol Ital 45 : 91–113.
[47] Schlagintweit, F; Rashidi, K. (2016). Some new and poorly known benthic foraminifera from late Maastrichtian shallow-water carbonates of the Zagros zone, SW Iran. Acta Palaeontol Roman 12 : 53–70.
[48] Loeblich, AR; Tappan, H. (1988). Foraminiferal genera and their classification. two volumes. New York: Van Nostrand Reinhold. p. 2047
[49] Brasier, MD. (1984). Some geometrical aspects of fusiform planispiral shape in larger foraminifera. J Micropalaeontol 3 : 11–6, Available from. DOI: http://dx.doi.org/10.1144/jm.3.1.11 Accessed 26 February 2021
[50] Vicedo, V; Aguilar, M; Caus, E; Hottinger, L. (2009). Fusiform and laterally compressed alveolinaceans (Foraminiferida) from both sides of the Late Cretaceous Atlantic. Neues Jahrb Geol Paläontol 253 : 229–47, Available from. DOI: http://dx.doi.org/10.1127/0077-7749/2009/0253-0229 Accessed 26 February 2021
[51] Hamaoui, M; Fourcade, E. (1973). Révision de Rhapydionimae (alveolinidae, foraminifères). Bulletin des Centres de Recherches Exploration – Production Elf-Aquitaine 7 : 361–435.
[52] Langer, MR. (1989). Distribution, Diversity and Functional Morphology of Benthic Foraminifera from Vulcano (Mediterranean Sea) In: Unpublished Ph.D. Thesis. Switzerland: University of Basel. p. 159
[53] Langer, MR. (1993). Epiphytic foraminifera. Mar Micropaleontol 20 : 235–65, Available from. DOI: http://dx.doi.org/10.1016/0377-8398(93)90035-V Accessed 26 February 2021
[54] Langer, MR; Frick, H; Silk, MT. (1998). Photophile and sciaphile foraminifera from Lavezzi Island, Corsica. Rev Paléobiol 17 : 525–30.
[55] Langer, EJ; Goldbeck, MR. (2009). Biogeographic provinces and patterns of diversity in selected upper Cretaceous (Santonian–Maastrichtian) larger foraminifera. SEPM (Society for Sedimentary Geology), pp. 187–232.
[56] De Castro, P. (1985). Pseudorhapydionina dubia (De Castro, 1965). Pseudorhapydionina laurinensis (De Castro, 1965). Pseudorhipidionina casertana (De Castro, 1965) In: Neumann, M, Schroeder, R R (eds.), Les grands Foraminifères du Crétacé moyen de la region méditerranéenne. Geobios, Mémoire spéciale 7 : 88–97.
[57] De Castro, P. (1971). Osservazioni su Raadshoovenia van den Bold, e i suoi col nuovo genere Scandonea (Foraminiferida, Miliolacea). Boll Soc Nat Napoli 80 : 161–235.
[58] Fleury, J-J. (1977). Deux Rhapydionininae (Foraminifères, Alveolinidae) d’affinités américaines dans le Crétacé supérieur de Grèce (zone de Gavrovo-Tripolitza). Rev de Micropaléontol 20 (2) : 77–90.
[59] Luperto Sinni, E; Ricchetti, G. (1978). Studio micropaleontologicostratigrafico di una successione carbonatica del Cretacèo superior rilevata nel sottosuolo delle Murge sud-orientali. Riv Italiana di Paleontologia e Stratigrafia 84 : 561–666.
[60] Fleury, J-J; Bignot, G; Blondeau, A; Poignant, A. (1985). Biogeographie de Foraminifères tethysiens du Sénonien a l’Eocène supérieur. Bull Soc Geol Fr I (5) : 757–70.
[61] Caffau, M; Plenicar, M; Pugliese, N; Drobne, K. (1998). Late Maastrichtian rudists and microfossils in the Karst region (NE Italy and Slovenia). Geobios 31 : 37–46, Available from. DOI: http://dx.doi.org/10.1016/s0016-6995(98)80063-6 Accessed 26 February 2021
[62] Goldbeck, EJ; Langer, MR. (2009). Biogeographic provinces and patterns of diversity in selected Upper Cretaceous (Santonian-Maastrichtian) larger foraminifera. SEPM (Society for Sedimentary Geology), SEPM Special Publication No. 93. pp. 187–232.
[63] Fourcade, É. (1966). Murciella cuvillieri n. gen. n. sp. nouveau foraminifère du Sénonien supérieur du sud-est de l’Espagne. Rev de Micropaléontol 9 : 147–55.
[64] Reichel, M. (1984). Le crible apertural de Rhapydionina liburnica Stache du Maastrichtien de Vremski-Britof, Yugoslavie In: Oertli, HJ (ed.), Benthos’ 83. Paris, France: Elf-Aquitaine, pp. 525–32.
[65] Fleury, J-J. (1979). Le genre Murciella (Foraminifère, Rhapydionininae), dans le Crétacé supérieur de Grèce (zone de Gavrovo-Tripolitza). Geobios 12 (2) : 149–85, Available from. DOI: http://dx.doi.org/10.1016/S0016-6995(79)80078-9 Accessed 26 February 2021
[66] Haynes, JR. (1981). Foraminifera. London: MacMillan. p. 433
[67] Hottinger, L. (2006/02). Illustrated glossary of terms used in foraminiferal research. Carnets de Géologie/Notebooks on Geology, Memoir, Available from. DOI: http://dx.doi.org/10.4267/2042/5832 Accessed 26 February 2021
[68] Drobne, K. (1974). Les grandes Miliolids des couches paleocenes de la Yougoslavie du Nord-Ouest ( Idalina, Fabularia, Lacazina, Periloculina). Razprave IV, razr, SAZU 17 : 129–84.
[69] Hottinger, L. (1997). Shallow benthic foraminiferal assemblages as signals for depth of their deposition and their limitations. Bull Soc Géol Fr 168/4 : 491–505.
[70] Hottinger, L. (1966). Foraminifères rotaliformes et Orbitoïdes du Sénonien Inférieur Pyrénéen. Eclogae Geol Helv 59 : 277–301.
[71] Caus, E; Hottinger, L. (1986). Particularidades de la fauna (foraminìferos) del Cretácico superior pirenaico. Paleontolgia ì Evolució 20 : 115–23.
[72] Caus, E. (1988). Upper Cretaceous larger foraminifera: paleoecological distribution. Rev Paléobiol 2 : 417–9.
[73] Miller, KG; Kominz, MA; Browning, JV; Wright, JD; Mountain, GS; Katz, ME. (2005). The phanerozoic record of global sea-level change. Science 310 : 1293–8, Available from. DOI: http://dx.doi.org/10.1126/science.1116412 Accessed 26 February 2021
[74] Bonet, F. (1956). Zonificacion microfaunistica de las calizas Cretacicas del este de Mexico. Boletín de la Asociación Mexicana de Geólogos Petroleros 8 : 3–102.
[75] Lucas, SG; Krainer, K. (2015). Cretaceous stratigraphy, paleontology, petrography, depositional environments, and cycle stratigraphy at Cerro de Cristo Rey, Doña Ana County. New Mexico Geology 32 : 103–30.
[76] Omaña, L; López-Doncel, R; Torres, JR; Alencaster, G; López-Caballero, I. (2019). Mid–late Cenomanian larger benthic foraminifers from the El Abra Formation W Valles-San Luis Potosi Platform, central–eastern Mexico: taxonomy, biostratigraphy and paleoenvironmental implications. Boletin de la Soc Geol Mex 71 : 691–725, Available from. DOI: http://dx.doi.org/10.18268/bsgm2019v71n3a5 Accessed 26 February 2021
[77] Conkin, JE; Conkin, BM. (1958). Revision of the genus Nummoloculina and emendation of Nummoloculina heimi Bonet. Micropaleontology 4 : 149–58, Available from. DOI: http://dx.doi.org/10.2307/1484300 Accessed 26 February 2021
[78] Fourcade, E; Tardy, M; Vila, JM. (1975). Streptalveolina mexicana n. gen. n. sp., un Alveolinidae nouveau (Foraminifère) du Cénomanien du Mexique. Rev de Micropaleontol 17 : 110–16.
[79] Scott, RW. (2002). Upper Albian benthic foraminifers new in West Texas. J Foraminiferal Re 32 : 43–50, Available from. DOI: http://dx.doi.org/10.2113/0320043 Accessed 26 February 2021
[80] Mancini, EA; Scott, RW. (2006). Sequence stratigraphy of comanchean cretaceous outcrop strata of Northeast and South-Central Texas: implications for enhanced petroleum exploration. Transactions Gulf Coast Association of Geological Societies 56 : 539–50.
[81] De Castro, P. (1983). Cisalveolina fraasi (Gümbel) Reichel: diffusione geografica e problemi stratigrafici. Boll Soc Nat Napoli 9091981 : 99–129.
[82] Omaña, L; López-Doncel, R; Torres-Hernández, JR; Alencáster, G. (2012). Biostratigraphy and paleoenvironment of the Cenomanian/Turonian boundary interval based on foraminifera from W Valles–San Luis Potosí Platform, Mexico. Micropaleontology 58 : 457–85.
[83] Jaillard, E; Arnaud-Vanneau, A. (1993). The Cenomanian–Turonian transition on the Peruvian margin. Cretac Res 14 : 585–605, Available from. DOI: http://dx.doi.org/10.1006/cres.1993.1041 Accessed 26 February 2021
[84] Fourcade, É; Fleury, J-J. (2001). Origine, evolution et systématique de Praechubbina n. gen., foraminifère Alveolinacea de Crétacé supérieur du Guatemala et du Mexique. Rev de Micropaleontol, Paris 44 : 125–57, Available from. DOI: http://dx.doi.org/10.1016/s0035-1598(01)90153-9 Accessed 26 February 2021
[85] Fleury, J-J. (2018). Rhapydioninidés du Campanien-Maastrichtien en region méditerranéenne: Les genres Murciella, Sigalveolina n. gen. et Cyclopseudedomia . Carnets de Géologie 18 (11) : 233–80, Available from. DOI: http://dx.doi.org/10.4267/2042/68382 Accessed 26 February 2021
[86] Omaña, L; Alencáster, G. (2019). Late-latest Maastrichtian foraminiferal assemblage from the Angostura Platform, Chiapas, SE Mexico: biostratigraphic, paleoenvironmental and paleobiogeographic significance. J S Am Earth Sci 91 : 203–13, Available from. DOI: http://dx.doi.org/10.1016/j.jsames.2019.02.004 Accessed 26 February 2021
[87] Hanzawa, S. (1967). Three New Tertiary Foraminiferal Genera from Florida, Saipan and Guam In: Transactions and Proceedings of the Palaeontological Society of Japan, New Series, no.65. : 19–25.
[88] Cole, WS. (1941). Stratigraphic and paleontologic studies of wells in Florida – no 1. Florida Geological Survey 19 : 91.
[89] Levin, HL. (1957). Micropaleontology of the Oldsmar Limestone (Eocene) of Florida. Micropaleontology 3 (2) : 137–54, Available from. DOI: http://dx.doi.org/10.2307/1484194 Accessed 26 February 2021
[90] Butterlin, J; Bonet, F. (1960). Microfauna del Eoceno Inferior de la Península de Yucatan. Paleontologia Mexicana, Instituto de Geología, Mexico 7 : 1–18.
[91] van den Bold, WA. (1946). Contribution to the study of Ostracoda with special reference to the tertiary and cretaceous microfauna of the Caribbean region. Amsterdam: DeBussy. p. 167
[92] Butterlin, J. (1981). Claves para la determinación de macroforaminíferos de Mexico y del Caribe del Cretacico Superior al Mioceno Medio. Instituto Mexicano de Petróleo, p. 29 pp. 1–51.
[93] Pêcheux, MJF. (1984). Le Senonien superieur-Tertiaire du Chiapas (S.E. Mexique) et ses Macroforaminiferes, These 3 e Cycle, Univ. Nice. p. 154
[94] Fleury, J-J; Fourcade, E. (1987). Un nouvel Alveolinidae du Paléocène – Eocene inférieur du Mexique. Ses affinités avec les genres Murciella et Yaberinella . Rev Micropaléontol 30 : 91–110.
[95] Powell, JB. (2010). Larger foraminiferal biostratigraphy, systematics and paleoenvironments of the Avon Park Formation and Ocala Limestone, Highlands County, Florida. Florida International University, ProQuest Dissertations Publishing, p. 3447806.
[96] Gold, DP; Fentona, JPG; Casas-Gallegoa, M; Novaka, V; Pérez-Rodrígueza, I; Ceteana, C. (2018). The biostratigraphic record of Cretaceous to Paleogene tectono-eustatic relative sea-level change in Jamaica. J South Am Earth Sci 86 : 140–61, Available from. DOI: http://dx.doi.org/10.1016/j.jsames.2018.06.011 Accessed 26 February 2021
[97] Robinson, E. (1974). Some larger foraminifera from the Eocene limestones at Red Gal Ring, Jamaica. Verh Naturforsch Ges Basel 84 (1) : 281–92.
[98] Robinson, E; Mitchell, SF. (1999). Middle Eocene to Oligocene stratigraphy and palaeogeography in Jamaica: a window on the Nicaragua Rise (UNESCO/IUGS IGCP 393). Contrib Geol UWI Mona 4 : 1–45.
[99] Robinson, E; Paytan, A; Chien, CT; Broach, K. (2018). Dating the White Limestone of Jamaica using Sr isotope stratigraphy: a progress report. Caribb J Earth Sci 49 : 11–21.
[100] Brasier, MD. (1995). Fossil indicators of nutrient levels. 1: Eutrophication and climate change. Geol Soc Spec Publ 83 : 113–32.
[101] Hottinger, L. (2007). Revision of the foraminiferal genus Globoreticulina RAHAGHI, 1978, and of its associated fauna of larger foraminifera from the late Middle Eocene of Iran. Notebooks on Geology. (Brest), CG2007-A06, : 1–51, Available from. DOI: http://dx.doi.org/10.4267/2042/9213 Accessed 26 February 2021
[102] Berggren, WA; Prothero, DR. (1992). Eocene–Oligocene climatic and biotic evolution: an overview In: Prothero, DR, Berggren, WA WA (eds.), Eocene–oligocene climatic and biotic evolution. Princeton: Princeton University Press, pp. 1–28.
[103] Smith, AG; Pickering, KT. (2003). Oceanic gateways as a critical factor to initiate icehouse earth. J Geol Soc London 160 : 337–40, Available from. DOI: http://dx.doi.org/10.1144/0016-764902-115 Accessed 26 February 2021
[104] Bassi, D; Loriga Broglio, C. (1999). Alveolinids and Middle-Upper Eocene boundary in Northeastern Italy (Veneto, Colli Berici). J Foraminifer Res 29 : 222–35.
[105] Bassi, D; Di Domenico, G; Pignatti, J; Abramovich, S; Hallock, P; Koenen, J. (2019). Data from: Palaeobiogeography and evolutionary patterns of the larger foraminifer Borelis de Montfort (Borelidae). UK: Papers in Palaeontology, Wiley Online Library, Available from. DOI: http://dx.doi.org/10.5061/dryad.st65n12 Accessed 26 February 2021
[106] BouDagher-Fadel, MK; Noujaim Clark, G. (2006). Stratigraphy, paleoenvironment and paleogeography of Maritime Lebanon: a key to Eastern Mediterranean Cenozoic history. Stratigraphy 3 : 81–118.
[107] Sirel, S. (2003). Foraminiferal description and biostratigraphy of the Bartonian, Priabonian and Oligocene shallow-water sediments of the southern and eastern Turkey. Rev Paleobiol 22 : 269–339.
[108] Di Carlo, M; Accordi, G; Carbone, F; Pignatti, J. (2010). Biostratigraphic analysis of Paleogene lowstand wedge conglomerates of a tectonically active platform margin (Zakynthos Islands, Greece). J Mediterr Earth Sci 2 : 31–92, Available from. DOI: http://dx.doi.org/10.3304/JMES.2010.004 Accessed 26 February 2021
[109] Benedetti, A. (2010). Biostratigraphic remarks on the Caltavuturo Formation (Eocene-Oligocene) cropping out at Portella Colla (Madonie Mts., Sicily). Rev Paléobiol 29 : 197–216.
[110] Braga, JC; Bassi, D. (2011). Facies and coralline algae from Oligocene limestones in the Malaguide Complex (SE Spain). Ann Nat Hist Wien Ser A 113 : 291–308.
[111] Drobne, K. (1988). Structural elements and stratigraphic distribution of larger Miliolids in the foraminiferal family Fabulariidae. Rev Paléobiol 2 : 643–61.
[112] Drobne, K; Ćosović, V. (2009). Palaeobiogeography of the Late Cretaceous to Paleogene larger miliolids from tropical to subtropical sea belts (Neotethys to Caribbean). Bull Soc géol Fr 180 : 317–31.
[113] Serra-Kiel, J; Vicedo, V; Razin, Ph; Grélaud, C. (2016). Selandian-Thanetian larger foraminifera from the lower Jafnayn Formation in the Sayq area (eastern Oman Mountains). Geol Acta 14 : 315–33, Available from. DOI: http://dx.doi.org/10.1344/GeologicaActa2016.14.3.7 Accessed 26 February 2021
[114] Drobne, K. (1985). Periloculina dalmatina, a new trematophorid miliolid from the Cuisian of Jugoslavia. Razprave 4 Razreda SAZU 26 : 159–76.
[115] Renema, W. (2002). Larger foraminifera and their distribution patterns on the Spermonde shelf, South Sulawesi. Scr Geol 124 : 1–263.
[116] Nuttall, WLF. (1925). The Stratigraphy of the Laki Series (Lower Eocene) of parts of Sind and Baluchistan (India); with a Description of the Larger Foraminifera contained in those beds. Q J Geol Soc 81 (1-4) : 417–53, Available from. DOI: http://dx.doi.org/10.1144/GSL.JGS.1925.081.01-04.18 Accessed 26 February 2021
[117] Lunt, P; Allan, T. (2004). Larger foraminifera in Indonesian biostratigraphy, calibrated to isotopic dating. Bandung: GRDC Museum Workshop on Micropaleontology. p. 109
[118] Matsumaru, K. (1974). Larger foraminifera from east Mindanao, the Philippines. Geol Paleontol Southeast Asia 14 : 101–15.
[119] Adams, CG. (1965). The foraminifera and stratigraphy of the Melinau Limestone, Sarawak, and its importance in Tertiary correlation. Q J Geol Soc London 121 : 283–338, Available from. DOI: http://dx.doi.org/10.1144/gsjgs.121.1.0283 Accessed 26 February 2021
[120] Hanzawa, S. (1947). Eocene foraminifera from Haha-Jima (Hillsborough Island). J Paleontol 21 : 254–9.
[121] Crespin, I. (1962). Lacazinella, a new genus of trematophore foraminifera. Micropaleontology 8 : 337–42.
[122] Lunt, P. (2003). Biogeography of some Eocene larger foraminifera, and their application in distinguishing geological plates. Palaeontol Electronica 6 (1) : 22. Available from: http://palaeo-electronica.org/paleo/2003_2/geo/issue2_03.htm . Accessed 26 February 2021
[123] Matsumaru, K. (1990). A new genus of the trematophorate miliolids (Fabulariidae) from the Eocene Setogawa group, Southwest Japan. Trans Proc Palaeontol Soc Jpn NS 160 : 663–73.
[124] Bassi, D; Braga, JC; Iryu, Y. (2009). Palaeobiogeographic patterns of a long living monophyletic lineage: Lithophyllum pustulatum species group (Corallinales, Rhodophyta). Palaeogeogr Palaeoclimatol Palaeoecol 284 : 237–45, Available from. DOI: http://dx.doi.org/10.1016/j.palaeo.2009.10.003 Accessed 26 February 2021
[125] Adams, CG; Gentry, AW; Whybrow, PJ. (1983). Dating the terminal Tethyan event. In: Meulenkamp JE, editor. Reconstruction of Marine Paleoenvironments . Utrecht Micropaleontological Bulletins 30 : 273–98.
[126] Betzler, C; Schmitz, S. (1997). First record of Borelis melo and Dendritina sp. in the Messinian of SE Spain (Cabo de Gata, Province Almeria). Paläont Z 71 : 211–6.
[127] Brandano, M; Tomassetti, L; Sardella, R; Tinelli, C. (2016). Progressive deterioration of trophic conditions in a carbonate ramp environment: the Lithothamnion Limestone, Majella Mountain (Tortonian-early Messinian, Central Apennines, Italy). Palaios 31 : 125–40, Available from. DOI: http://dx.doi.org/10.2110/palo.2015.022 Accessed 26 February 2021
[128] Eames, FE; Banner, FT; Blow, WH; Clarke, WJ. (1962). Fundamentals of mid-tertiary stratigraphical correlation. Cambridge: Cambridge University Press. p. 163
[129] Bignot, G; Guernet, G. (1976). Sur la présence de Borelis curdica (Reichel) dans le Miocéne de l’ile de Kos (Greece). Géol Médit 3 : 15–26.
[130] Buchbinder, B; Martinotti, GM; Simantov, R; Zilberman, E. (1993). Temporal and spatial relationships in Miocene reef carbonates in Israel. Palaeogeogr Palaeoclimatol Palaeoecol 101 : 97–116, Available from. DOI: http://dx.doi.org/10.1016/0031-0182(93)90154-B Accessed 26 February 2021
[131] Cahuzac, B. (1997). Poignant A. Essai de biozonation de l’Oligo-Miocène dans les bassins européens à l’aide des grands foraminifères néritiques. Bull Soc Géol Fr 168 : 155–69.
[132] Cicha, I; Rögl, F; Čtyroká, J; Rupp, Ch; Bajraktarevic, Z; Baldi, T. (1998). Oligocene, miocene foraminifera of the central paratethys. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft, 549. Frankfurt am Main: W. Kramer, pp. 1–325.
[133] Jones, RJ; Simmons, M. (2006). On the stratigraphical and palaeobiogeographical significance of Borelis melo melo (Fichtel & Moll, 1798) and B. melo curdica (Reichel, 1937) (Foraminifera, Miliolida, Alveolinidae). J Micropalaeontol 25 : 175–85.
[134] Hyams, O; Almogi-Labin, A; Benjamini, C. (2002). Larger foraminifera of the southeastern Mediterranean shallow continental shelf off Israel. Isr J Earth Sci 51 : 169–79.
[135] Nunes, AL; Katsanevakis, S; Zenetos, A; Cardoso, AC. (2014). Gateways to alien invasions in the European seas. Aquat Invasions 9 : 133–44, Available from. DOI: http://dx.doi.org/10.3391/ai.2014.9.2.02 Accessed 26 February 2021
[136] Haunold, TG; Baal, C; Piller, WE. (1997). Benthic foraminiferal associations in the Northern Bay of Safaga, Red Sea, Egypt. Mar Micropaleontol 29 : 185–210, Available from. DOI: http://dx.doi.org/10.1016/S0377-8398(96)00031-X Accessed 26 February 2021
[137] Von Rögl, F. (1998). Palaeogeographic considerations for Mediterranean and Paratethys Seaways (oligocene to Miocene). Ann Nat Hist Mus Wien 99A : 279–310.
[138] Langer, MR; Hottinger, L. (2000). Biogeography of selected ‘larger’ foraminifera. Micropaleontology 46 : 105–26.
[139] Cole, WS. (1954). Larger foraminifera from Guam. J Paleontol 13 : 183–89.
[140] Hottinger, L; Halicz, E; Reiss, Z. (1993). Recent foraminifera from the Gulf of Aqaba, Red Sea. SAZU 33 : 1–179.
[141] Cole, WS. (1957). Larger foraminifera from Eniwetok Atoll. U S Geol Surv Prof Pap 260 : 743–84.
[142] Matsumaru, K. (1996). Tertiary larger foraminifera (Foraminiferida) from the Ogasawara Islands, Japan. Paleontol Soc Jpn Spec Pap 36 : 1–239.
[143] Javaux, EJ; Scott, DB. (2003). Illustration of modern benthic foraminifera from Bermuda and remarks on distribution in other subtropical/tropical areas. Palaeontologia Electronica 6 (4) : 29.
[144] Langer, MR; Lipps, JH. (2003). Foraminiferal distribution and diversity, Madang Reef and Lagoon, Papua New Guinea. Coral Reefs 22 : 143–54, Available from. DOI: http://dx.doi.org/10.1007/s00338-003-0298-1 Accessed 26 February 2021
[145] Förderer, M; Langer, MR. (2018). Atlas of benthic Foraminifera from coral reefs of the Raja Ampat Archipelago (Irian Jaya, Indonesia). Micropaleontology 64 (1-2) : 1–170.
[146] Wignall, PB; Benton, MJ. (1999). Lazarus taxa and fossil abundance at times of biotic crisis. J Geol Soc 156 (3) : 453–56, Bibcode:1999JGSoc.156.453W. Available from. DOI: http://dx.doi.org/10.1144/gsjgs.156.3.0453 Accessed 26 February 2021
[147] Adams, CG. (1967). Tertiary foraminifera in the Tethyan, American, and Indo-Pacific Provinces. In: Adams CG, Ager, D, editors. Aspects of tethyan biogeography, systematics association . London: Publication, special publication 7 : 195–217.
[148] Rosen, BR. (1988). Progress, problems and patterns in the biogeography of reef corals and other tropical marine organisms. Helgoländer Meeresuntersuchungen 42 : 269–301, Available from. DOI: http://dx.doi.org/10.1007/BF02366046 Accessed 26 February 2021
[149] Benedetti, A; Marino, M; Pichezzi, MR. (2018). Paleocene to lower Eocene larger foraminiferal assemblages from Central Italy: new remarks on biostratigraphy. Riv Ital Paleontol Stratigr 124 : 73–90, Available from. DOI: http://dx.doi.org/10.13130/2039-4942/9627 Accessed 26 February 2021
[150] Özcan, E; Mitchell, SF; Less, G; Robinson, E; Bryan, JR; Pignatti, J. (2019). A revised suprageneric classification of American orthophragminids with emphasis on late Paleocene representatives from Jamaica and Alabama. J Syst Palaeontol 17 : 1551–79, Available from. DOI: http://dx.doi.org/10.1080/14772019.2018.1539778 Accessed 26 February 2021